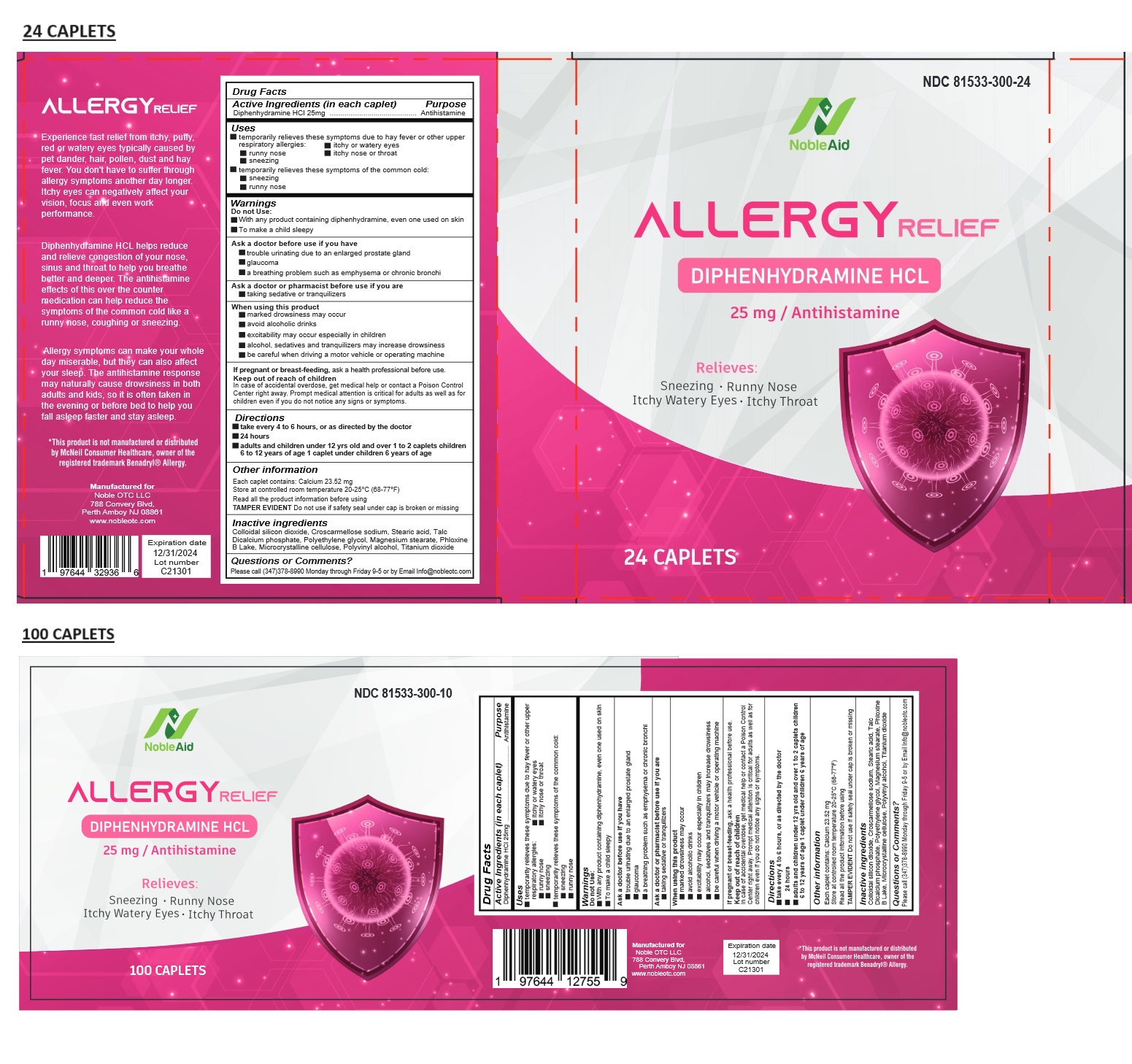
Label: NOBLEAID ALLERGY RELIEF- diphenhydramine hydrochloride tablet
- NDC Code(s): 81533-300-10, 81533-300-24
- Packager: Noble OTC LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients (in each caplet)
- Purpose
- Uses
-
Warnings
Do not Use:
• With any product containing diphenhydramine, even one used on skin
• To make a child sleepyAsk a doctor before use if you have
• trouble urinating due to an enlarged prostate gland
• glaucoma
• a breathing problem such as emphysema or chronic bronchiAsk a doctor or pharmacist before use if you are
• taking sedative or tranquilizersWhen using this product
• marked drowsiness may occur
• avoid alcoholic drinks
• excitability may occur especially in children
• alcohol, sedatives and tranquilizers may increase drowsiness
• be careful when driving a motor vehicle or operating machineIf pregnant or breast-feeding, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
Experience fast relief from itchy, puffy, red or watery eyes typically caused by pet dander, hair, pollen, dust and hay fever. You don't have to suffer through allergy symptoms another day longer. Itchy eyes can negatively affect your vision, focus and even work performance.
Diphenhydramine HCL helps reduce and relieve congestion of your nose, sinus and throat to help you breathe better and deeper. The antihistamine effects of this over the counter medication can help reduce the symptoms of the common cold like a runny nose, coughing or sneezing.
Allergy symptoms can make your whole day miserable, but they can also affect your sleep. The antihistamine response may naturally cause drowsiness in both adults and kids, so it is often taken in the evening or before bed to help you fall asleep faster and stay asleep
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Benadryl® Allergy.
Manufactured for
Noble OTC LLC
788 Convery Blvd,
Perth Amboy NJ 08861
www.nobleotc.com - Packaging
-
INGREDIENTS AND APPEARANCE
NOBLEAID ALLERGY RELIEF
diphenhydramine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81533-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C RED NO. 27 (UNII: 2LRS185U6K) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape OVAL Size 11mm Flavor Imprint Code D25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81533-300-24 24 in 1 BLISTER PACK; Type 0: Not a Combination Product 07/01/2023 2 NDC:81533-300-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/01/2023 Labeler - Noble OTC LLC (041836435)