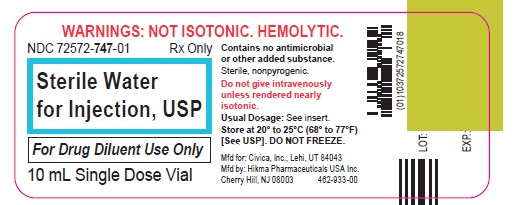
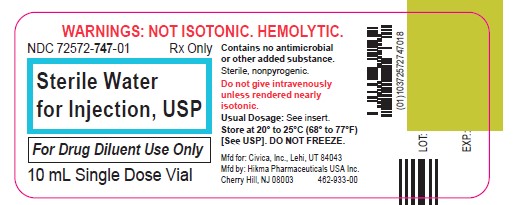
Label: STERILE WATER- water injection, solution
- NDC Code(s): 72572-747-01, 72572-747-10
- Packager: Civica, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
This preparation is designed solely for parenteral use only after addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection.
Sterile Water for Injection, USP is a sterile, nonpyrogenic preparation of water for injection which contains no bacteriostat, antimicrobial agent or added buffer and is supplied only in single-dose containers to dilute or dissolve drugs for injection. For intravenous injection, add sufficient solute to make an approximately isotonic solution.
Water for Injection, USP is chemically designated H2O.
The glass vial is a Type I borosilicate glass and meets the requirements according to the USP standards.
-
CLINICAL PHARMACOLOGY
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water for distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
The small volume of fluid provided by Sterile Water for Injection, USP when used only as a pharmaceutic aid for diluting or dissolving drugs for parenteral injection, is unlikely to exert a significant effect on fluid balance except possibly in neonates or very small infants.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Do not use for intravenous injection unless the osmolar concentration of additives results in an approximate isotonic admixture.
Consult the manufacturer’s instructions for choice of vehicle, appropriate dilution or volume for dissolving the drugs to be injected, including the route and rate of injection.
Inspect reconstituted (diluted or dissolved) drugs for clarity (if soluble) and freedom from unexpected precipitation or discoloration prior to administration.
Pregnancy
Animal reproduction studies have not been conducted with Sterile Water for Injection. It is also not known whether sterile water containing additives can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sterile Water for Injection with additives should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness have been established in pediatric patients. However, in neonates or very small infants the volume of fluid may affect fluid and electrolyte balance.
Drug Interactions
Some drugs for injection may be incompatible in a given vehicle, or when combined in the same vehicle or in a vehicle containing benzyl alcohol. Consult with pharmacist, if available.
Use aseptic technique for single or multiple entry and withdrawal from all containers.
When diluting or dissolving drugs, mix thoroughly and use promptly.
Do not store reconstituted solutions of drugs for injection unless otherwise directed by the manufacturer of the solute.
Do not use unless the solution is clear and seal intact. Do not reuse single-dose containers. Discard unused portion.
-
ADVERSE REACTIONS
Reactions which may occur because of this solution, added drugs or the technique of reconstitution or administration include febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasation.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate countermeasures, and if possible, retrieve and save the remainder of the unused vehicle for examination.
-
OVERDOSAGE
Use only as a diluent or solvent. This parenteral preparation is unlikely to pose a threat of fluid overload except possibly in neonates or very small infants. In the event these should occur, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS, PRECAUTIONS and ADVERSE REACTIONS.
-
DOSAGE AND ADMINISTRATION
The volume of the preparation to be used for diluting or dissolving any drug for injection is dependent on the vehicle concentration, dose and route of administration as recommended by the manufacturer.
This parenteral should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Sterile Water for Injection, USP is supplied in the following:
10 mL single dose vials packaged in cartons of 10 vials (NDC 72572-747-10)Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature.] DO NOT FREEZE.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured for:
Civica, Inc.
Lehi, Utah 84043Manufactured by:
Hikma Pharmaceuticals USA Inc.
Cherry Hill, NJ 08003Revised: December 2021
462-932-00
- PRINCIPAL DISPLAY PANEL
- SERIALIZATION IMAGE
-
INGREDIENTS AND APPEARANCE
STERILE WATER
water injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72572-747 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72572-747-10 10 in 1 CARTON 02/23/2022 1 NDC:72572-747-01 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206369 02/23/2022 Labeler - Civica, Inc. (081373942) Registrant - Hikma Pharmaceuticals USA Inc. (946499746) Establishment Name Address ID/FEI Business Operations Hikma Pharmaceuticals USA Inc. 946499746 analysis(72572-747) , label(72572-747) , manufacture(72572-747) , pack(72572-747)