Label: LINCOMYCIN-SPECTINOMYCIN WATER SOLUBLE POWDER- lincomycin-spectinomycin powder, for solution
- NDC Code(s): 23243-4240-2
- Packager: Huvepharma, Inc
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated May 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- GENERAL PRECAUTIONS
-
DOSAGE & ADMINISTRATION
This packet contains:
Lincomycin hydrochloride, equivalent to lincomycin ...... 16.7 grams
Spectinomycin dihydrochloride pentahydrate,
equivalent to spectinomycin.................33.3 grams
Total Antibiotic Activity 50.0 gramsApproved by FDA under ANADA # 200-407
NET WEIGHT: 2.65 oz (75 grams)Manufactured for Huvepharma, Inc.
Peachtree City, Georgia 30269
Made in Bulgaria® Registered Trademark of Huvepharma, Inc.
LOT
EXP.
-
INDICATIONS & USAGE
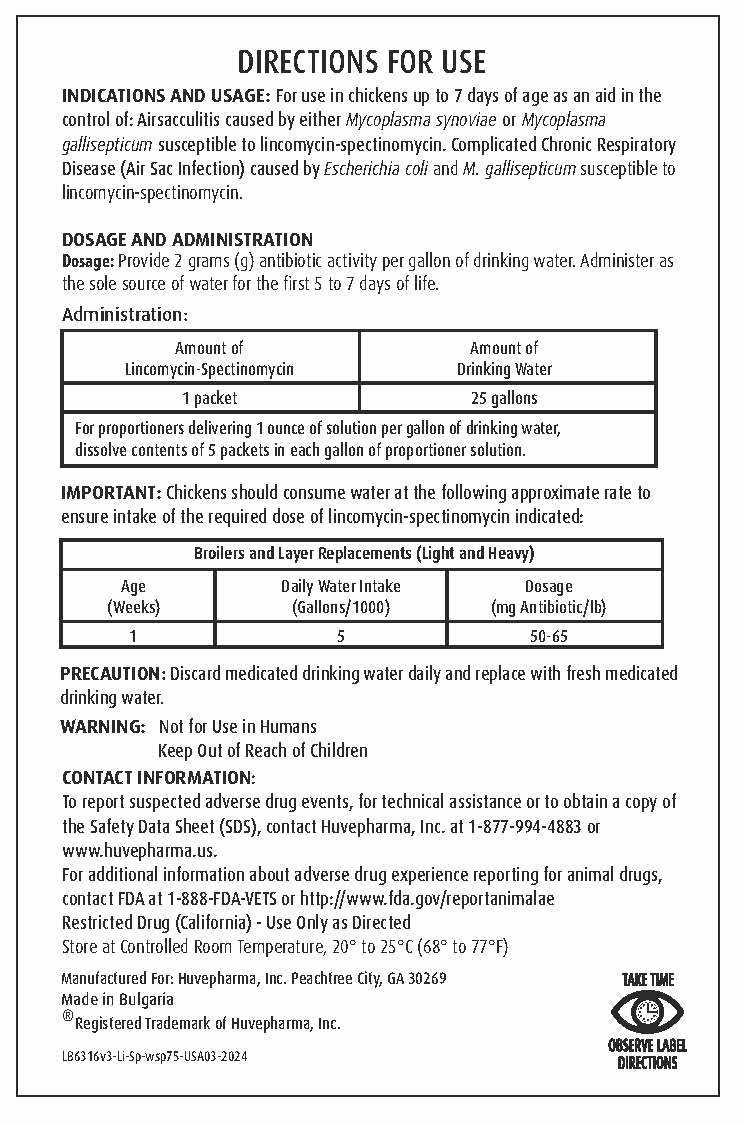
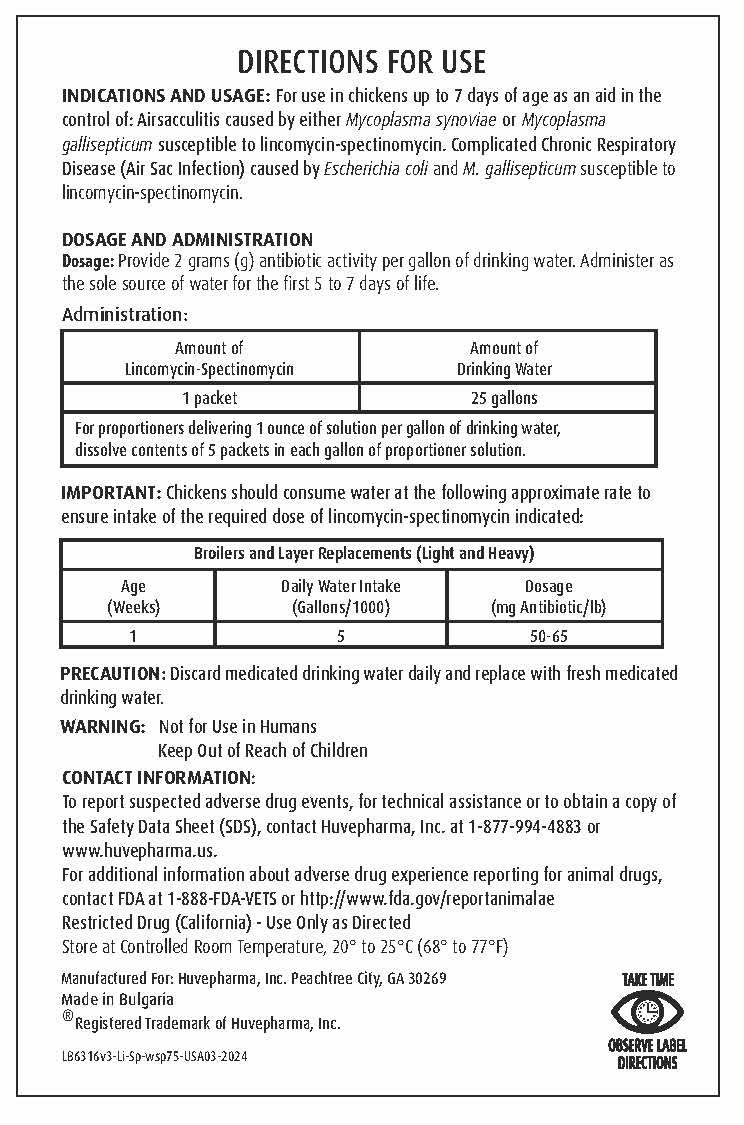
DIRECTIONS FOR USE
INDICATIONS AND USAGE: For use in chickens up to 7 days of age as an aid in the control of:
Airsacculitis caused by either Mycoplasma synoviae or Mycoplasma gallisepticum susceptible to lincomycin-spectinomycin.
Complicated Chronic Respiratory Disease (Air Sac Infection) caused by Escherichia coli and M. gallisepticum susceptible
to lincomycin-spectinomycin. -
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Dosage: Provide 2 grams (g) antibiotic activity per gallon of drinking water. Administer as the sole source of water for the first 5 to 7 days of life.Administration:
Amount of Lincomycin-Spectinomycin Amount of Drinking Water 1 packet 25 gallons For proportioners delivering 1 ounce of solution per gallon of drinking water,
dissolve contents of 5 packets in each gallon of proportioner solution.IMPORTANT: Chickens should consume water at the following approximate rate to ensure intake of the required dose of lincomycin-spectinomycin indicated:
Broilers and Layer Replacements (Light and Heavy) Age
(Weeks)Daily Water Intake
(Gallons/1000)Dosage
(mg Antibiotic/lb)1 5 50-65 - GENERAL PRECAUTIONS
-
USER SAFETY WARNINGS
WARNING: Not for Use in Humans
Keep Out of Reach of ChildrenCONTACT INFORMATION:
To report suspected adverse drug events, for technical assistance or to obtain a
copy of the Safety Data Sheet (SDS), contact Huvepharma, Inc. at 1-877-994-4883
or www.huvepharma.us.For additional information about adverse drug experience reporting for animal drugs,
contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae - STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LINCOMYCIN-SPECTINOMYCIN WATER SOLUBLE POWDER
lincomycin-spectinomycin powder, for solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:23243-4240 Route of Administration Oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LINCOMYCIN HYDROCHLORIDE (UNII: M6T05Z2B68) (LINCOMYCIN - UNII:BOD072YW0F) LINCOMYCIN 16.7 g in 75 g SPECTINOMYCIN HYDROCHLORIDE (UNII: HWT06H303Z) (SPECTINOMYCIN - UNII:93AKI1U6QF) SPECTINOMYCIN 33.3 g in 75 g Inactive Ingredients Ingredient Name Strength Sucrose (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-4240-2 75 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200407 11/01/2022 Labeler - Huvepharma, Inc (619153559) Registrant - Huvepharma EOOD (552671651)