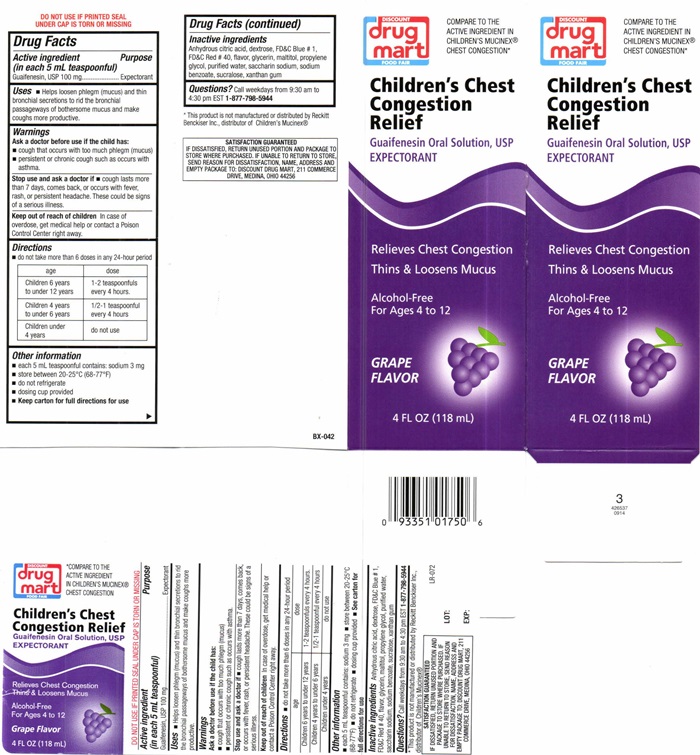
Label: CHILDRENS CHEST CONGESTION RELIEF GRAPE- guaifenesin liquid
- NDC Code(s): 53943-511-24
- Packager: Discount Drug Mart
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug FactsActive ingredient(in each 5 mL teaspoonful)
- Purpose
- Keep out of reach of children
- Uses
- Warnings
- Stop use and as a doctor if
- Directions
- Other information
- Inactive ingredients
- Questions?
-
DISCOUNT drug mart FOOD FAIR Childrens Chest Congestion Relief - Grape
COMPARE TO THE ACTIVE INGREDIENT IN CHILDREN'S MUCINEX® CHEST CONGESTION
DISCOUNT
drug
mart
FOOD FAIR
Children's Chest Congestion Relief
Guaifenesin Oral Solution, USP
EXPECTORANT
Relieves Chest Congestion
Thins & Loosens Mucus
Alcohol-Free
For Ages 4 to 12
Grape Flavor
4 FL OZ (118 mL)
* This product is not manufactured or distributed by Reckitt Benckiser, Inc., distributer of Children's Mucinex®
SATISFACTION GUARANTEED
IF DISSASIFIED , RETURN UNUSED PORTION AND PACKAGE TO STORE WHERE PURCHASED. IF UNABLE TO RETURN TO STORE, SEND REASON FOR DISSATISFACTION, NAME, ADDRESS AND EMPTY PACKAGE TO: DISCOUNT DRUG MART, 211 COMMERCE DRIVE, MEDINA, OHIO 44256
res

-
INGREDIENTS AND APPEARANCE
CHILDRENS CHEST CONGESTION RELIEF GRAPE
guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-511 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DEXTROSE (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) MALTITOL (UNII: D65DG142WK) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor grape (GRAPE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-511-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 02/04/2015 Labeler - Discount Drug Mart (047741335)