Label: MARCAINE- bupivacaine hydrochloride and epinephrine bitartrate injection, solution
- NDC Code(s): 0362-0557-05
- Packager: Septodont, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
Description
Bupivacaine hydrochloride is (±) -1-Butyl-2´, 6´-pipecoloxylidide monohydrochloride, monohydrate, a white crystalline powder that is freely soluble in 95 percent ethanol, soluble in water, and slightly soluble in chloroform or acetone. It has the following structural formula:
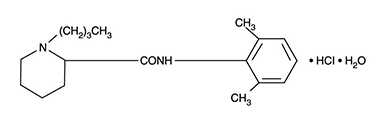 Molecular Weight - 342.90 C18H28N2O • HCl • H2O
Molecular Weight - 342.90 C18H28N2O • HCl • H2O
Epinephrine bitartrate is (-)-1-(3,4-Dihydroxyphenyl)-2-methylamino-ethanol (+) tartrate (1:1) salt. It has the following structural formula:
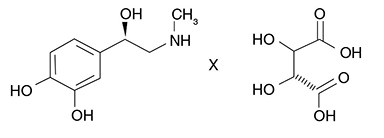 Molecular Weight - 333.29 C13H19NO9
Molecular Weight - 333.29 C13H19NO9
Bupivacaine is available in a sterile isotonic solution with epinephrine 1:200,000 (as bitartrate). Solutions of bupivacaine containing epinephrine may not be autoclaved.
Bupivacaine is related chemically and pharmacologically to the aminoacyl local anesthetics. It is a homologue of mepivacaine and is chemically related to lidocaine. All three of these anesthetics contain an amide linkage between the aromatic nucleus and the amino or piperidine group. They differ in this respect from the procaine-type local anesthetics, which have an ester linkage. -
CLINICAL PHARMACOLOGY
Bupivacaine stabilizes the neuronal membrane and prevents the initiation and transmission of nerve impulses, thereby effecting local anesthesia.
The onset of action following dental injections is usually 2 to 10 minutes and anesthesia may last two or three times longer than lidocaine and mepivacaine for dental use, in many patients up to 7 hours. The duration of anesthetic effect is prolonged by the addition of epinephrine 1:200,000.
It has also been noted that there is a period of analgesia that persists after the return of sensation, during which time the need for strong analgesic is reduced.
After injection of bupivacaine for caudal, epidural or peripheral nerve block in man, peak levels of bupivacaine in the blood are reached in 30 to 45 minutes, followed by a decline to insignificant levels during the next three to six hours. Because of its amide structure, bupivacaine is not detoxified by plasma esterases but is detoxified, via conjugation with glucuronic acid, in the liver. When administered in recommended doses and concentrations, bupivacaine does not ordinarily produce irritation or tissue damage.
Systemic absorption of local anesthetics produces effects on the cardiovascular and central nervous systems (CNS). At blood concentrations achieved with normal therapeutic doses, changes in cadiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. Recent clinical reports and animal research suggest that these cardiovascular changes are more likely to occur after unintended intravascular injection of bupivacaine. Therefore, incremental dosing is necessary.
Following systemic absorption, local anesthetics can produce central nervous system stimulation, depression, or both. Apparent central stimulation is manifested as restlessness, tremors and shivering progressing to convulsions, followed by depression and coma progressing ultimately to respiratory arrest. However, the local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited state.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
LOCAL ANESTHETICS SHOULD BE EMPLOYED ONLY BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE EMERGENCIES WHICH MIGHT ARISE FROM THE BLOCK TO BE EMPLOYED, AND THEN ONLY AFTER INSURING THE IMMEDIATE AVAILABILITY OF OXYGEN, OTHER RESUSCITATIVE DRUGS, CARDIOPULMONARY RESUSCITATIVE EQUIPMENT, AND THE PERSONNEL RESOURCES NEEDED FOR PROPER MANAGEMENT OF TOXIC REACTIONS AND RELATED EMERGENCIES. (See also ADVERSE REACTIONS and PRECAUTIONS.) DELAY IN PROPER MANAGEMENT OF DOSE-RELATED TOXICITY, UNDERVENTILATION FROM ANY CAUSE, AND/OR ALTERED SENSITIVITY MAY LEAD TO THE DEVELOPMENT OF ACIDOSIS, CARDIAC ARREST AND, POSSIBLY, DEATH.
Small doses of local anesthetics injected into the head and neck area, as small as nine to eighteen milligrams, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Confusion, convulsions, respiratory depression, and/or respiratory arrest, cardiovascular stimulation or depression and cardiac arrest have been reported. Reactions resulting in fatalities have occurred on rare occasions. In a few cases, resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded (see DOSAGE AND ADMINISTRATION).
It is essential that aspiration for blood or cerebrospinal fluid (where applicable) be done prior to injecting any local anesthetic, both the original dose and all subsequent doses, to avoid intravascular injection. However, a negative aspiration does not ensure against an intravascular injection.
Reactions resulting in fatality have occurred on rare occasions with the use of local anesthetics, even in the absence of a history of hypersensitivity.
This solution, which contains a vasoconstrictor, should be used with extreme caution for patients whose medical history and physical evaluation suggest the existence of hypertension, arteriosclerotic heart disease, cerebral vascular insufficiency, heart block, thyrotoxicosis and diabetes, etc., as well as patients receiving drugs likely to produce alterations in blood pressure.
Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Marcaine® and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene
blue, exchange transfusion, or hyperbaric oxygen.Marcaine® 0.5% (5 mg/mL) with epinephrine 1:200,000 injection or other vasopressors should not be used concomitantly with ergot-type oxytocic drugs, because a severe persistent hypertension may occur. Likewise, solutions of bupivacaine containing a vasoconstrictor, such as epinephrine, should be used with extreme caution in patients receiving monoamine oxidase inhibitors (MAOI) or antidepressants of the triptyline or imipramine types, because severe prolonged hypertension may result.
Until further experience is gained in children younger than 12 years, administration of bupivacaine in this age group is not recommended.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
-
PRECAUTIONS
The safety and effectiveness of local anesthetics depend upon proper dosage, correct technique, adequate precautions, and readiness for emergencies.
The lowest dosage that gives effective anesthesia should be used in order to avoid high plasma levels and serious systemic side effects. Injection of repeated doses of bupivacaine may cause significant increase in blood levels with each additional dose, due to accumulation of the drug or its metabolites or due to slow metabolic degradation. Tolerance varies with the status of the patient.
Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with age and physical condition.
Because of the long duration of anesthesia, when bupivacaine with epinephrine is used for dental injections, patients should be cautioned about the possibility of inadvertent trauma to tongue, lips, and buccal mucosa and advised not to chew solid foods or test the anesthetized area by biting or probing.
Changes in sensorium, such as excitation, disorientation, drowsiness, may be early indications of a high blood level of the drug and may occur following inadvertent intravascular administration or rapid absorption of bupivacaine.
Solutions containing a vasoconstrictor should be used cautiously in areas with limited blood supply, in the presence of diseases that may adversely affect the patient's cardiovascular system, or in patients with peripheral vascular disease.
Caution is advised in administration of repeat doses of bupivacaine to patients with severe liver disease.
Local anesthetic procedures should be used with caution when there is inflammation and/or sepsis in the region of the proposed injection.
Drug Interactions
See WARNINGS concerning solutions containing a vasoconstrictor.
If sedatives are employed to reduce patient apprehension, use reduced doses, since local anesthetic agents, like sedatives, are central nervous system depressants which in combination may have an additive effect.
Marcaine® 0.5% (5 mg/mL) with epinephrine 1:200,000 injection should be used cautiously in persons with known drug allergies or sensitivities, particularly to the amide-type local anesthetics. Serious dose-related cardiac arrhythmias may occur if preparations containing a vasoconstrictor such as epinephrine are employed in patients during or following the administration of chloroform, halothane, cyclopropane, trichloroethylene, or other related agents. In deciding whether to use these products concurrently in the same patient, the combined action of both agents upon the myocardium, the concentration and volume of vasoconstrictor used, and the time since injection, when applicable, should be taken into account.
Information for Patients/Patient Counseling Information
When appropriate, the dentist should discuss information including adverse reactions in the package insert for Marcaine® 0.5% (5 mg/mL) with epinephrine 1:200,000 injection. Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Clinically Significant Drug Interactions
The administration of local anesthetic solutions containing epinephrine or norepinephrine to patients receiving mono-amine oxidase inhibitors or tricyclic antidepressants may produce severe, prolonged hypertension. Concurrent use of these agents should generally be avoided. In situations when concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs and of ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.
Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine.
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
EXAMPLES OF DRUGS ASSOCIATED WITH METHEMOGLOBINEMIA: Class Examples Nitrates/Nitrites nitric oxide, nitroglycerin, nitroprusside, nitrous oxide Local anesthetics articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine Antineoplastic Agents cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase Antibiotics dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides Antimalarials chloroquine, primaquine Anticonvulsants phenobarbital, phenytoin, sodium valproate Other drugs acetaminophen, metoclopramide, quinine, sulfasalazine Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of bupivacaine hydrochloride have not been conducted. The mutagenic potential and the effect on fertility of bupivacaine hydrochloride has not been determined.
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Bupivacaine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Bupivacaine hydrochloride produced development toxicity when administered subcutaneously to pregnant rats and rabbits at clinically relevant doses. This does not exclude the use of bupivacaine at term for obstetrical anesthesia or analgesia.
Bupivacaine hydrochloride was administered subcutaneously to rats at doses of 4.4, 13.3, and 40 mg/kg and to rabbits at doses of 1.3, 5.8, and 22.2 mg/kg during the period of organogenesis (implantation to closure of the hard palate). The high doses are approximately 4-times the daily maximum recommended human dose (MRHD) of 90 mg/day on a mg dose/m2 body surface area (BSA) basis. No embryo-fetal effects were observed in rats at the high dose which caused increased maternal lethality. An increase in embryo-fetal deaths was observed in rabbits at the high dose in the absence of maternal toxicity with the fetal No Observed Adverse Effect Level being a comparable dose to the MRHD on a BSA basis.
In a rat pre- and post-natal development study (dosing from implantation through weaning) conducted at subcutaneous doses of 4.4, 13.3 and 40 mg/kg mg/kg/day, decreased pup survival was observed at the high dose. The high dose is approximately 4-time the daily MRHD of 90 mg/day on a BSA basis.
-
ADVERSE REACTIONS
Reactions to Marcaine® 0.5% (5 mg/mL) with epinephrine 1:200,000 injection are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, inadvertent intravascular injection or slow metabolic degradation.
Excessive plasma levels of the amide-type local anesthetics cause systemic reactions involving the central nervous system and the cardiovascular system. The central nervous system effects are characterized by excitation or depression. The first manifestation may be nervousness, dizziness, blurred vision, or tremors, followed by drowsiness, convulsions, unconsciousness, and possibly respiratory arrest. Since excitement may be transient or absent, the first manifestation may be drowsiness, sometimes merging into unconsciousness and respiratory arrest. Other central nervous system effects may be nausea, vomiting, chills, constriction of the pupils, or tinnitus. The cardiovascular manifestations of excessive plasma levels may include depression of the myocardium, blood pressure changes (usually hypotension), and cardiac arrest. Allergic reactions, which may be due to hypersensitivity, idiosyncrasy, or diminished tolerance, are characterized by cutaneous lesions (e.g., urticaria), edema, and other manifestations of allergy. Detection of sensitivity by skin testing is of doubtful value. Transient facial swelling and puffiness may occur near the injection site.
Treatment of Reactions
Toxic effects of local anesthetics require symptomatic treatment; there is no specific cure. The dentist should be prepared to maintain an airway and to support ventilation with oxygen and assisted or controlled respiration as required. Supportive treatment of the cardiovascular system includes intravenous fluids and, when appropriate, vasopressors (preferably those that stimulate the myocardium). Convulsions may be controlled with oxygen and intravenous administration, in small increments, of a barbiturate, as follows: preferably, an ultra-short-acting barbiturate such as thiopental or thiamylal; if this is not available, a short-acting barbiturate (e.g., secobarbital or pentobarbital) or diazepam. Intravenous barbiturates or anticonvulsant agents should only be administered by those familiar with their use.
-
DOSAGE AND ADMINISTRATION
As with all anesthetics, the dosage varies and depends upon the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, individual tolerance, and the technique of anesthesia. The lowest dosage needed to provide effective anesthesia should be administered. For specific techniques and procedures, refer to standard textbooks.
The 0.5% concentration with epinephrine is recommended for infiltration and block injection in the maxillary and mandibular area when a longer duration of local anesthetic action is desired, such as for oral surgical procedures generally associated with significant postoperative pain. The average dose of 1.8 mL (9 mg) per injection site will usually suffice; an occasional second dose of 1.8 mL (9 mg) may be used if necessary to produce adequate anesthesia after making allowance for 2 to 10 minutes onset time (see CLINICAL PHARMACOLOGY). The lowest effective dose should be employed and time should be allowed between injections; it is recommended that the total dose for all injection sites, spread out over a single dental sitting, should not ordinarily exceed 90 mg for a healthy adult patient (ten 1.8 mL injections of bupivacaine with epinephrine). Injections should be made slowly and with frequent aspirations. Until further experience is gained, bupivacaine in dentistry is not recommended for children younger than 12 years.
Parental drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard unused portion.
-
HOW SUPPLIED
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from light. Do not permit to freeze.
Marcaine® 0.5% (5 mg/mL) with epinephrine 1:200,000 injection, (as bitartrate)–Sterile isotonic solutions containing sodium chloride. Each 1 mL contains 5 mg bupivacaine hydrochloride and 0.0091 mg epinephrine bitartrate, with 0.5 mg sodium metabisulfite, 7 mg sodium chloride, 0.001 mL monothioglycerol, and 2 mg ascorbic acid as antioxidants, 0.0017 mL 60% sodium lactate buffer, and 0.1 mg edetate calcium disodium as stabilizer. The pH of these solutions is adjusted with sodium hydroxide or hydrochloric acid. Solutions of bupivacaine that contain epinephrine should not be autoclaved and should be protected from light. Do not use the solution if its colour is pinkish or darker than slightly yellow or if it contains a precipitate.
Marcaine® 0.5% (5 mg/mL) with epinephrine 1:200,000 injection (NDC 0362-0557-05) is available in cartons containing 5 blisters of 10 X 1.8 mL single-dose dental cartridges.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1.8 mL Cartridge Carton
-
INGREDIENTS AND APPEARANCE
MARCAINE
bupivacaine hydrochloride and epinephrine bitartrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0362-0557 Route of Administration SUBMUCOSAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE ANHYDROUS (UNII: AKA908P8J1) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 mL epinephrine bitartrate (UNII: 30Q7KI53AK) (epinephrine - UNII:YKH834O4BH) epinephrine 0.005 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium metabisulfite (UNII: 4VON5FNS3C) 0.5 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 7 mg in 1 mL monothioglycerol (UNII: AAO1P0WSXJ) 0.001 mL in 1 mL ascorbic acid (UNII: PQ6CK8PD0R) 2 mg in 1 mL sodium lactate (UNII: TU7HW0W0QT) 0.0017 mL in 1 mL edetate calcium disodium (UNII: 25IH6R4SGF) 0.1 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0362-0557-05 50 in 1 CARTON 06/12/2018 1 1.8 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077250 06/12/2018 Labeler - Septodont, Inc. (627058738) Registrant - Novocol Pharmaceutical of Canada, Inc. (201719960) Establishment Name Address ID/FEI Business Operations Novocol Pharmaceutical of Canada, Inc. 201719960 manufacture(0362-0557)


