Label: VITAL VAPOR- camphor, menthol ointment
- NDC Code(s): 70722-229-05
- Packager: Little Moon Essentials
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 30, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
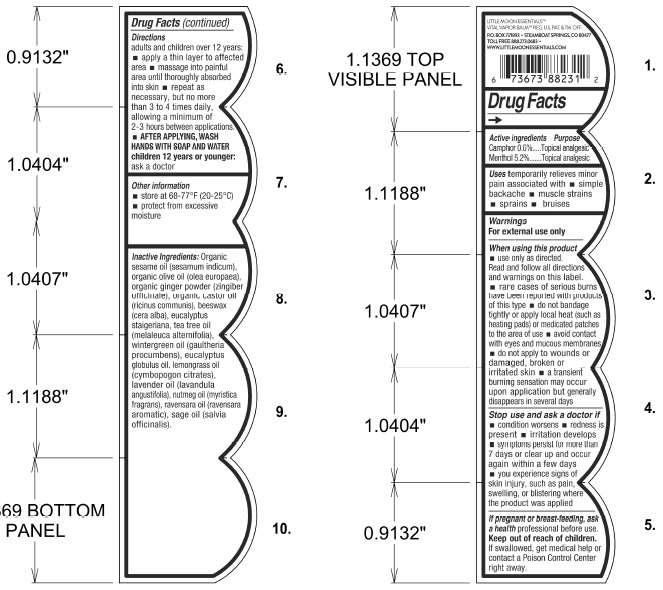
- Drug Facts
- Active Ingredients
- Purpose
- Uses
-
Warning
For exteral use only
When using this product
- use only as directed. Read and follow all directions and warnings on this label
- rare cases of serious burns have been reported with products of this type
- do not bandage tightly or apply local heat (such as heating pads) or medicated patches to teh area of use
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- a trasient burning sensation may occur upon application but generally disappears in several days
- Stop use and ask a doctor
- If pregnant or breast feeding
- Keep out of reach of children
-
Directions
Adults and children over 12 years:
- Apply a thin layer to affected area
- Massage into painful area until thoroughly absorbed into skin
- Repeat as necessary, but no more than 3 to 4 times daily, allowing a minimum of 2-3 hours between applications
- AFTER APPLYING, WASH HANDS WITH SOAP AND WATER
Children 12 years or younger: ask a doctor
- Other information
-
Inactive Ingredients
Organic sesame oil (sesamum indicum)
organic olive oil (olea europaea)
organic ginger powder (zingiber officinale)
organic castor oil (ricinus communis)
beeswax (cera alba)
eucaliptus staigeriana
tea tree oil (melaleuca alternifolia)
wintergreen oil (gaultheria procumbens)
eucalyptus globulus oil
lemongrass oil (cymbopogon citratus)
lavender oil (lavandula angustifolia)
nutmeg oil (myristica fragrans)
ravensara oil (ravensara aromatic)
sage oil (salvia officinalis)
- Principle Display Panel 14g container
-
INGREDIENTS AND APPEARANCE
VITAL VAPOR
camphor, menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70722-229 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 5.2 g in 100 g CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 0.6 g in 100 g Inactive Ingredients Ingredient Name Strength WEST INDIAN LEMONGRASS OIL (UNII: 5BIA40E9ED) NUTMEG OIL (UNII: Z1CLM48948) SAGE OIL (UNII: U27K0H1H2O) TEA TREE OIL (UNII: VIF565UC2G) METHYL SALICYLATE (UNII: LAV5U5022Y) EUCALYPTUS OIL (UNII: 2R04ONI662) LAVENDER OIL (UNII: ZBP1YXW0H8) CRYPTOCARYA AGATHOPHYLLA LEAF OIL (UNII: XM00Z00H98) OLIVE OIL (UNII: 6UYK2W1W1E) GINGER (UNII: C5529G5JPQ) CASTOR OIL (UNII: D5340Y2I9G) YELLOW WAX (UNII: 2ZA36H0S2V) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) SESAME OIL (UNII: QX10HYY4QV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70722-229-05 14 g in 1 CONTAINER; Type 0: Not a Combination Product 10/02/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/02/2016 Labeler - Little Moon Essentials (840835115) Registrant - Little Moon Essentials LLC (840835115) Establishment Name Address ID/FEI Business Operations Little Moon Essentials LLC 840835115 manufacture(70722-229)