Label: CLOTRIMAZOLE cream
- NDC Code(s): 45802-434-01, 45802-434-11
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
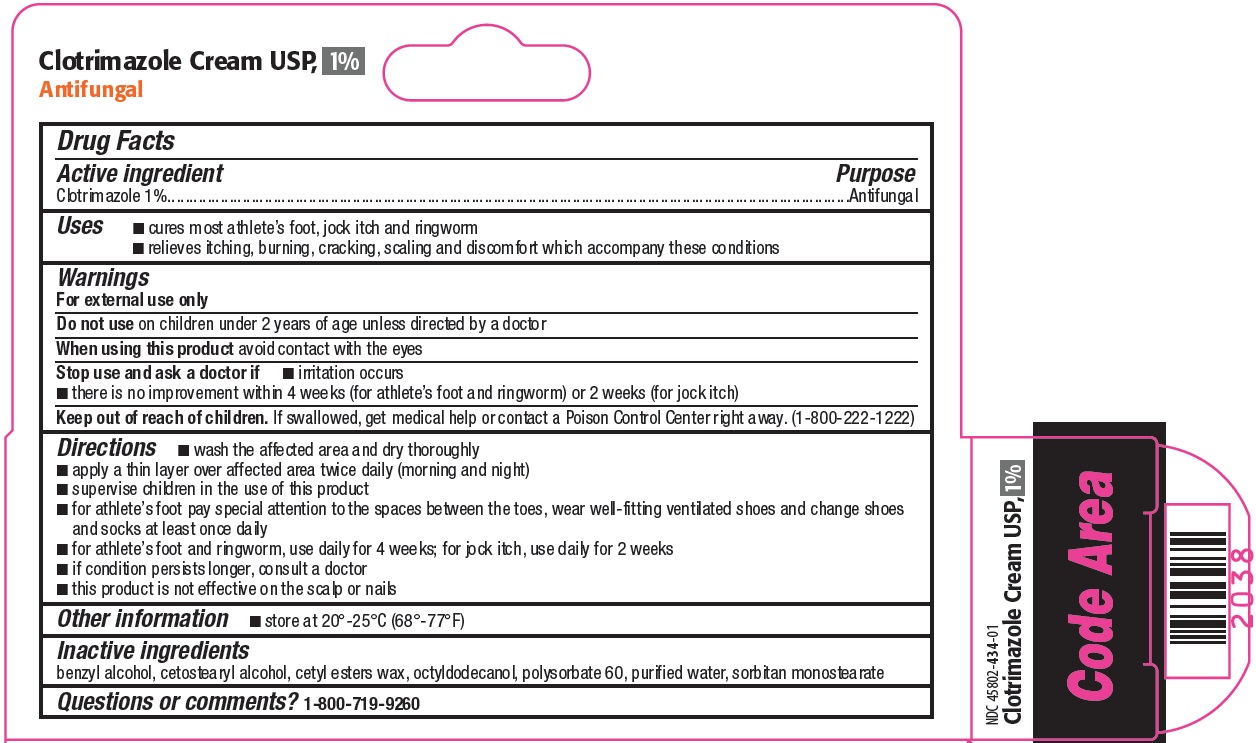
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- •
- wash the affected area and dry thoroughly
- •
- apply a thin layer over affected area twice daily (morning and night)
- •
- supervise children in the use of this product
- •
- for athlete’s foot pay special attention to the spaces between the toes, wear well-fitting ventilated shoes and change shoes and socks at least once daily
- •
- for athlete’s foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- •
- if condition persists longer, consult a doctor
- •
- this product is not effective on the scalp or nails
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE
clotrimazole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45802-434 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 1 g in 100 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL ESTERS WAX (UNII: D072FFP9GU) OCTYLDODECANOL (UNII: 461N1O614Y) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-434-01 1 in 1 CARTON 06/03/2011 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:45802-434-11 1 in 1 CARTON 06/09/2011 2 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 06/03/2011 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)