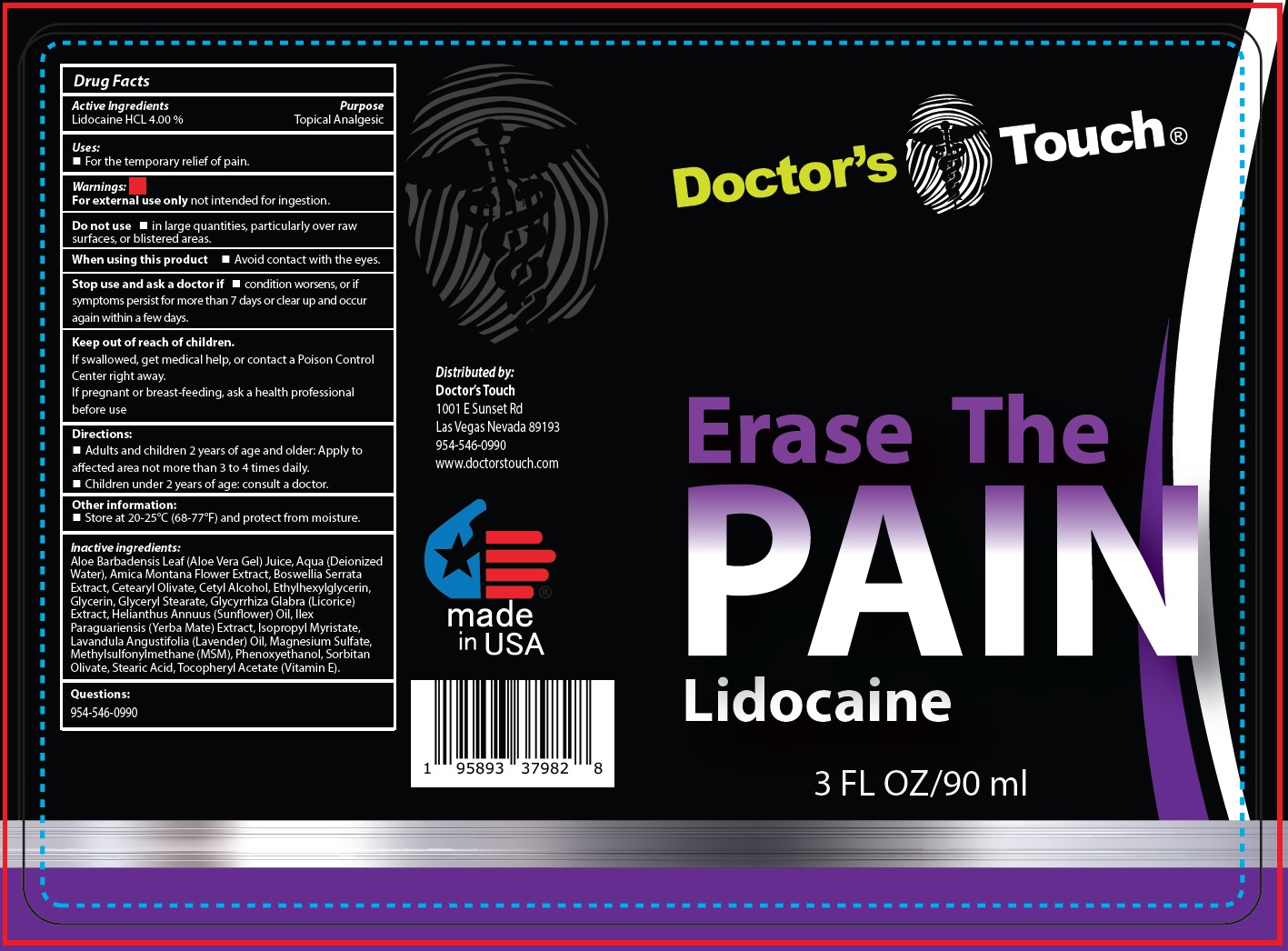
Label: DOCTORS TOUCH ERASE THE PAIN LIDOCAINE- lidocaine hydrochloride liquid
- NDC Code(s): 80045-400-00
- Packager: DOCTOR'S CHOICE MEDICAL, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses:
- Warnings:
- Directions:
- Other information:
-
Inactive ingredients:
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Amica Montana Flower Extract, Boswellia Serrata Extract, Cetearyl Olivate, Cetyl Alcohol, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Extract, Helianthus Annuus (Sunflower) Oil, Ilex Paraguariensis (Yerba Mate) Extract, lsopropyl Myristate, Lavandula Angustifolia (Lavender) Oil, Magnesium Sulfate, Methylsulfonylmethane (MSM), Phenoxyethanol, Sorbitan Olivate, Stearic Acid, Tocopheryl Acetate (Vitamin E).
- Questions:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
DOCTORS TOUCH ERASE THE PAIN LIDOCAINE
lidocaine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80045-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CETEARYL OLIVATE (UNII: 58B69Q84JO) CETYL ALCOHOL (UNII: 936JST6JCN) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LAVENDER OIL (UNII: ZBP1YXW0H8) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PHENOXYETHANOL (UNII: HIE492ZZ3T) SORBITAN OLIVATE (UNII: MDL271E3GR) STEARIC ACID (UNII: 4ELV7Z65AP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80045-400-00 90 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/17/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 04/17/2023 Labeler - DOCTOR'S CHOICE MEDICAL, LLC (081045011)