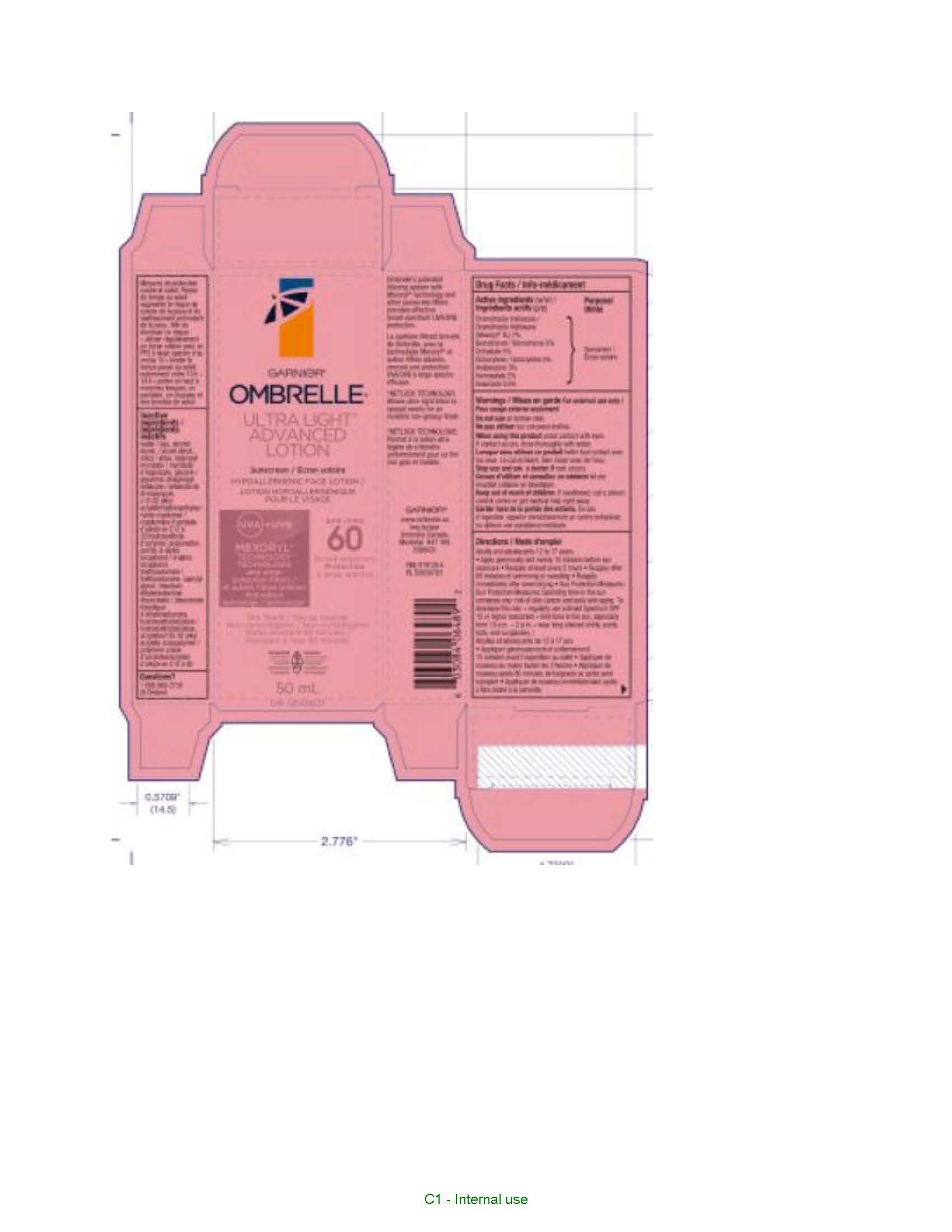
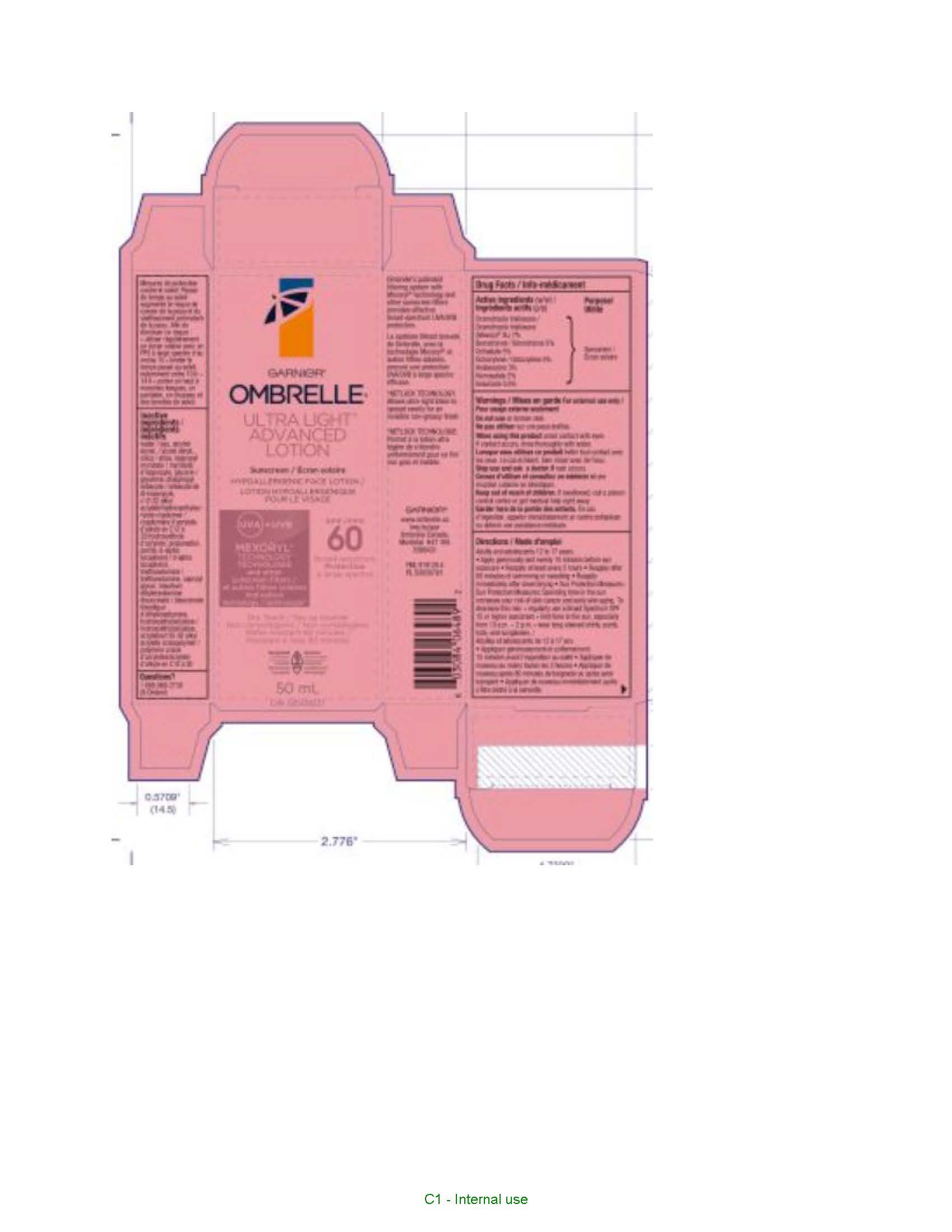
Label: GARNIER OMBRELLE ULTRA LIGHT ADVANCED 60 FACE SUNSCREEN- drometrizole trisiloxane, octocrylene, bemotrizinol, octisalate, avobenzone, homosalate and ensulizole lotion
- NDC Code(s): 49967-046-01
- Packager: L'OREAL USA PRODUCTS INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated December 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Warnings
-
Directions
Adults and adolescents 12 to 17 years.
Apply generously and evenly 15 minutes before sun exposure. Reapply at least every 2 hours. Reapply after 80 minutes of swimming or sweating. Reapply immediately after towel drying. Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk: regularly use a Broad Spectrum SPF 15 or higher sunscreen. Limit time in the sun, especially from 10 a.m. – 2 p.m. Wear long-sleeve shirts, pants, hats and sunglasses. -
Inactive ingredients
wwater, alcohol denat., silica, isopropyl myristate, glycerin, diisopropyl sebacate, propanediol, c12-22 alkyl acrylate/hydroxyethylacrylate copolymer, perlite, tocopherol, triethanolamine, caprylyl glycol, hydroxyethylcellulose, acrylates/c10-30 alkyl acrylate crosspolymer, pentylene glycol, butylene glycol, citric acid
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GARNIER OMBRELLE ULTRA LIGHT ADVANCED 60 FACE SUNSCREEN
drometrizole trisiloxane, octocrylene, bemotrizinol, octisalate, avobenzone, homosalate and ensulizole lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-046 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL DROMETRIZOLE TRISILOXANE (UNII: HC22845I1X) (DROMETRIZOLE TRISILOXANE - UNII:HC22845I1X) DROMETRIZOLE TRISILOXANE 70 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 20 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 50 mg in 1 mL BEMOTRIZINOL (UNII: PWZ1720CBH) (BEMOTRIZINOL - UNII:PWZ1720CBH) BEMOTRIZINOL 50 mg in 1 mL ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) GLYCERIN (UNII: PDC6A3C0OX) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) PROPANEDIOL (UNII: 5965N8W85T) PERLITE (UNII: 0SG101ZGK9) TOCOPHEROL (UNII: R0ZB2556P8) TROLAMINE (UNII: 9O3K93S3TK) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) PENTYLENE GLYCOL (UNII: 50C1307PZG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-046-01 1 in 1 CARTON 01/01/2023 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 01/01/2023 Labeler - L'OREAL USA PRODUCTS INC (002136794) Establishment Name Address ID/FEI Business Operations Dimensional Merchandising Inc. 076693183 manufacture(49967-046) , pack(49967-046)