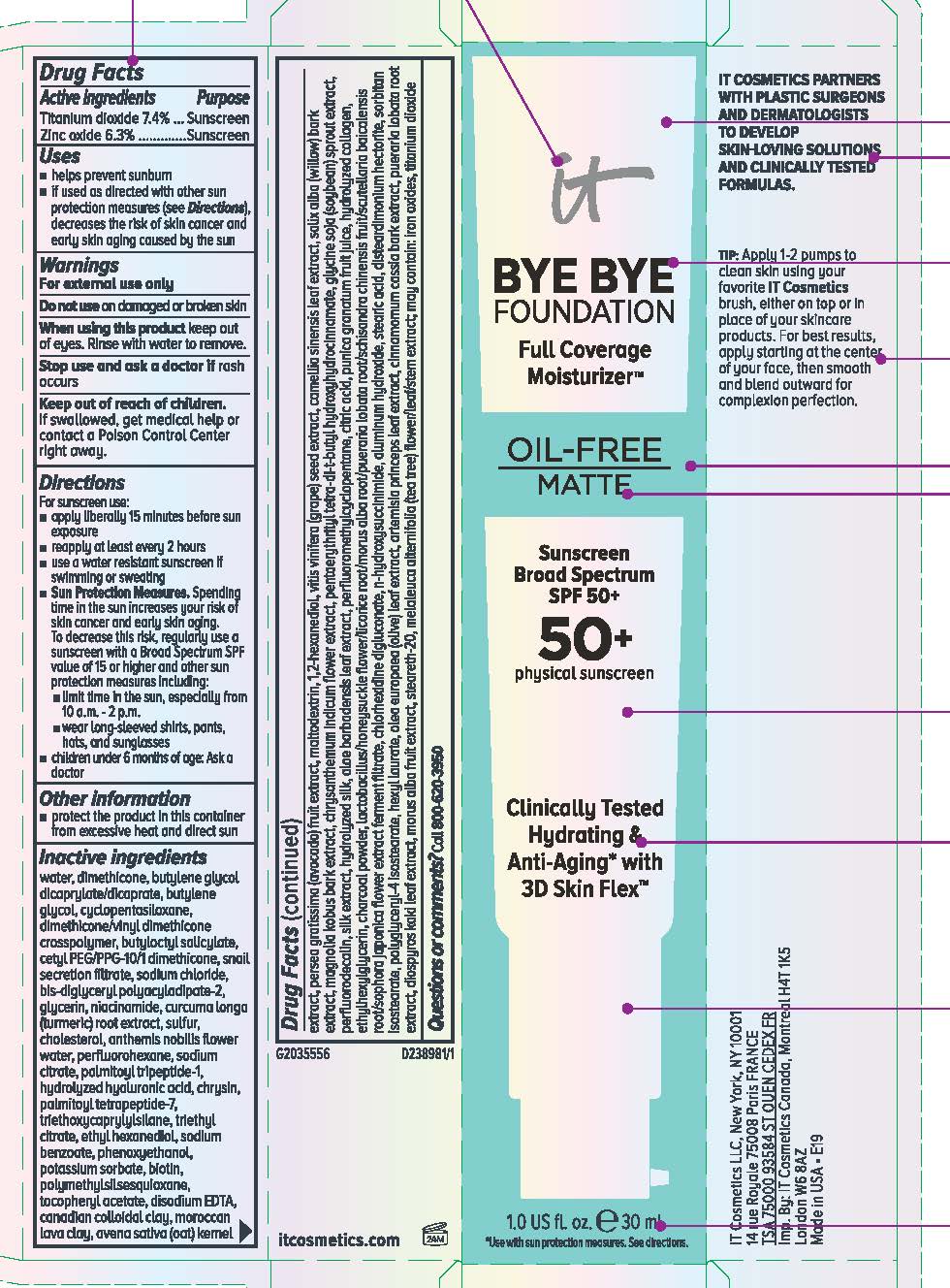
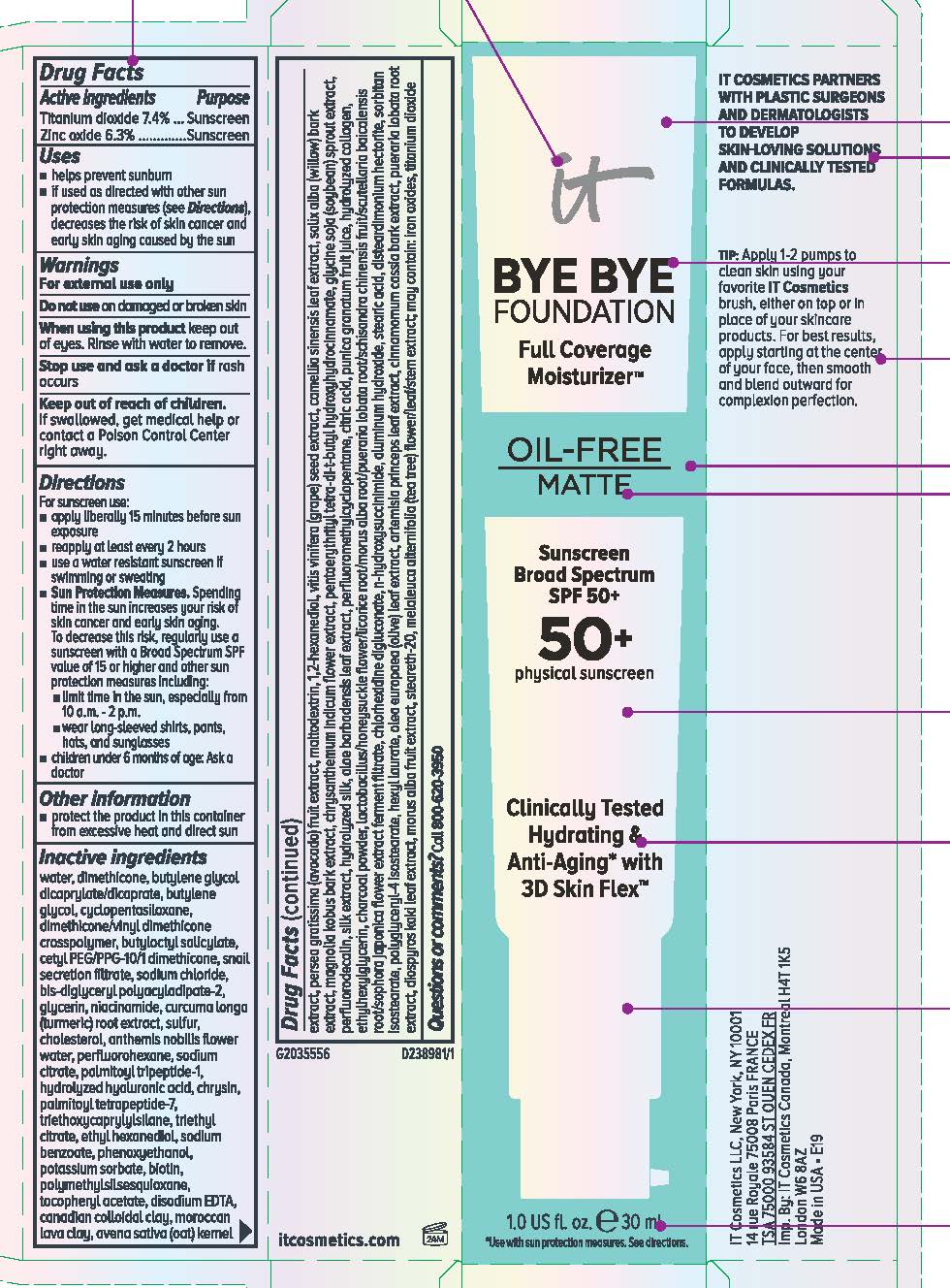
Label: IT COSMETICS BYE BYE FOUNDATION FULL COVERAGE MOISTURIZER OIL FREE MATTE BROAD SPECTRUM 50 PLUS SUNSCREEN- titanium dioxide and zinc oxide lotion
- NDC Code(s): 69259-4244-1
- Packager: IT COSMETICS, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
● shake well before use
● apply liberally 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk,
regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, dimethicone, butylene glycol dicaprylate/dicaprate, butylene glycol, cyclopentasiloxane, dimethicone/vinyl dimethicone crosspolymer, butyloctyl salicylate, cetyl PEG/PPG-10/1 dimethicone, snail secretion filtrate, sodium chloride, bis-diglyceryl polyacyladipate-2, aluminum hydroxide, stearic acid, disteardimonium hectorite, sorbitan isostearate, polyglyceryl-4 isostearate, hexyl laurate, triethoxycaprylylsilane, triethyl citrate, ethyl hexanediol, sodium benzoate, phenoxyethanol, potassium sorbate, polymethylsilsesquioxane, tocopheryl acetate, disodium EDTA, canadian colloidal clay, moroccan lava clay, avena sativa (oat) kernel extract, glycerin, niacinamide, curcuma longa (turmeric) root extract, sulfur, cholesterol, anthemis nobilis flower water, perfluorohexane, persea gratissima (avocado) fruit extract, maltodextrin, 1,2-hexanediol, vitis vinifera (grape) seed extract, camellia sinensis leaf extract, salix alba (willow) bark extract, magnolia kobus bark extract, chrysanthemum indicum flower extract, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, glycine soja (soybean) sprout extract, perfluorodecalin, silk extract, hydrolyzed silk, aloe barbadensis leaf extract, perfluoromethylcyclopentane, citric acid, olea europaea (olive) leaf extract, artemisia princeps leaf extract, cinnamomum cassia bark extract, pueraria lobata root extract, diospyros kaki leaf extract, morus alba fruit extract, steareth-20, melaleuca alternifolia (tea tree) flower/leaf/stem extract, punica granatum fruit juice, hydrolyzed collagen, ethylhexylglycerin, charcoal powder, lactobacillus/honeysuckle flower/licorice root/morus alba root/pueraria lobata root/schisandra chinensis fruit/scutellaria baicalensis root/sophora japonica flower extract ferment filtrate, chlorhexidine digluconate, n-hydroxysuccinimide, sodium citrate, palmitoyl tripeptide-1, hydrolyzed hyaluronic acid, chrysin, palmitoyl tetrapeptide-7, biotin; may contain: iron oxides, titanium dioxide
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IT COSMETICS BYE BYE FOUNDATION FULL COVERAGE MOISTURIZER OIL FREE MATTE BROAD SPECTRUM 50 PLUS SUNSCREEN
titanium dioxide and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69259-4244 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 74 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) SODIUM CHLORIDE (UNII: 451W47IQ8X) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) ETHOHEXADIOL (UNII: M9JGK7U88V) SODIUM BENZOATE (UNII: OJ245FE5EU) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) OAT (UNII: Z6J799EAJK) GLYCERIN (UNII: PDC6A3C0OX) NIACINAMIDE (UNII: 25X51I8RD4) TURMERIC (UNII: 856YO1Z64F) SULFUR (UNII: 70FD1KFU70) CHOLESTEROL (UNII: 97C5T2UQ7J) CHAMAEMELUM NOBILE FLOWER OIL (UNII: UB27587839) PERFLEXANE (UNII: FX3WJ41CMX) AVOCADO (UNII: SDS87L369F) MALTODEXTRIN (UNII: 7CVR7L4A2D) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) VITIS VINIFERA SEED (UNII: C34U15ICXA) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SALIX ALBA BARK (UNII: 205MXS71H7) MAGNOLIA KOBUS BARK (UNII: 54LVP49595) CHRYSANTHEMUM INDICUM FLOWER (UNII: I6OER6U04L) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) SOYBEAN (UNII: L7HT8F1ZOD) PERFLUNAFENE (UNII: 54A06VV62N) ALOE VERA LEAF (UNII: ZY81Z83H0X) PERFLUOROMETHYLCYCLOPENTANE (UNII: 8S014W4T75) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) ARTEMISIA PRINCEPS LEAF (UNII: SY077EW02G) CHINESE CINNAMON (UNII: WS4CQ062KM) PUERARIA MONTANA VAR. LOBATA ROOT (UNII: PET93F4I3C) DIOSPYROS KAKI LEAF (UNII: Q71GF9OBNO) WHITE MULBERRY (UNII: MN25R0HH5A) STEARETH-20 (UNII: L0Q8IK9E08) MELALEUCA ALTERNIFOLIA FLOWERING TOP (UNII: 5AZ4S6N66F) POMEGRANATE JUICE (UNII: 99S671U9KB) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) SODIUM CITRATE (UNII: 1Q73Q2JULR) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) CHRYSIN (UNII: 3CN01F5ZJ5) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) BIOTIN (UNII: 6SO6U10H04) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69259-4244-1 1 in 1 CARTON 06/01/2019 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2019 Labeler - IT COSMETICS, LLC (962591793) Establishment Name Address ID/FEI Business Operations Beauty Manufacturing Solutions Corp. 783200723 manufacture(69259-4244)