Label: FLORIVA- vitamin a acetate, .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol, thiamine, riboflavin, niacinamide, pyridoxine, levomefolate glucosamine, folic acid, cyanocobalamin, biotin, zinc gluconate, cupric oxide, and sodium fluoride tablet, chewable
- NDC Code(s): 52796-173-12, 52796-173-90
- Packager: BonGeo Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
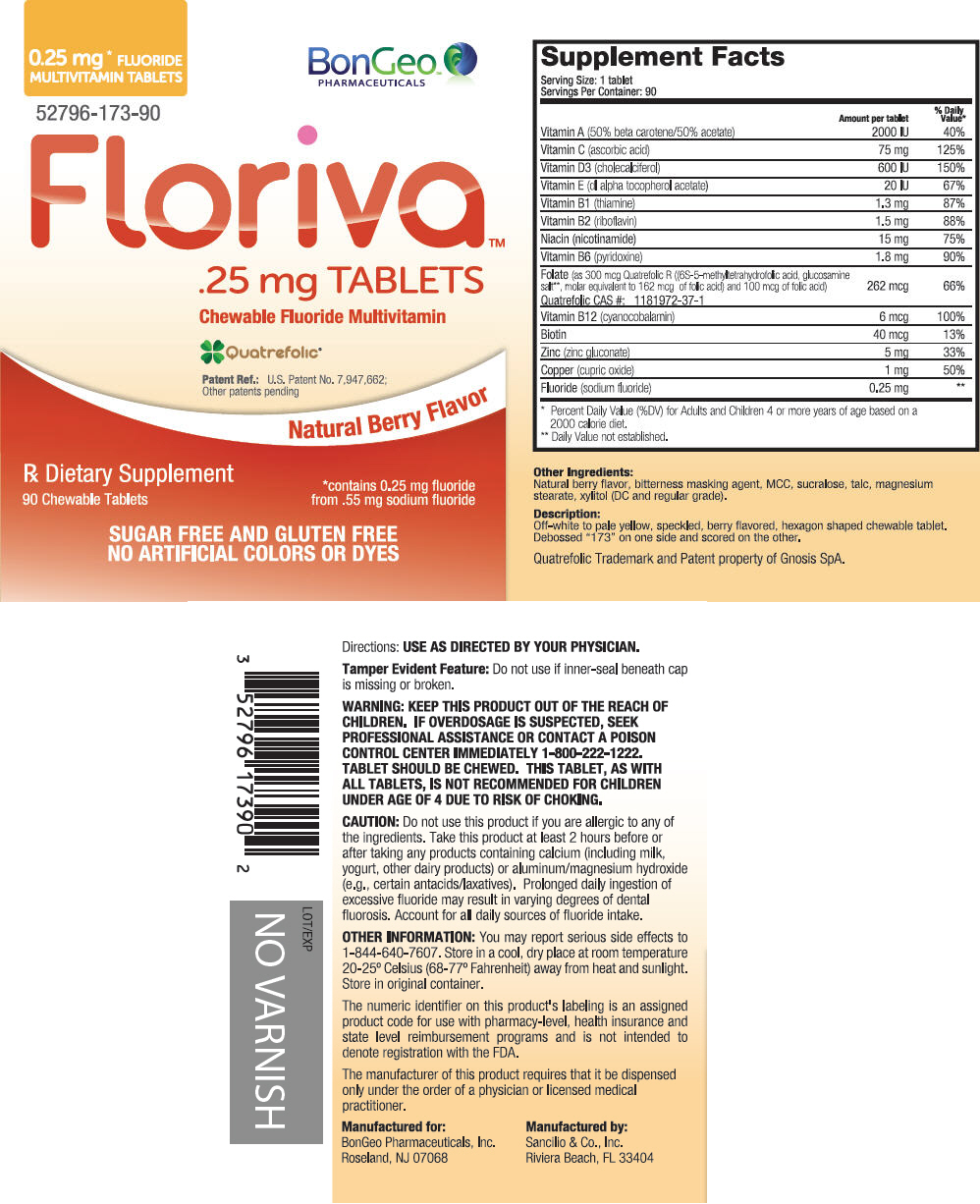
Supplement Facts Serving Size: 1 tablet
Servings Per Container: 90Amount per tablet % Daily Value* Vitamin A (50% beta carotene/50% acetate) 2000 IU 40% Vitamin C (ascorbic acid) 75 mg 125% Vitamin D3 (cholecalciferol) 600 IU 150% Vitamin E (dl alpha tocopherol acetate) 20 IU 67% Vitamin B1 (thiamine) 1.3 mg 87% Vitamin B2 (riboflavin) 1.5 mg 88% Niacin (nicotinamide) 15 mg 75% Vitamin B6 (pyridoxine) 1.8 mg 90% Folate (as 300 mcg Quatrefolic R ((6S-5-methyltetrahydrofolic acid, glucosamine salt†, molar equivalent to 162 mcg of folic acid) and 100 mcg of folic acid)
Quatrefolic CAS #: 1181972-37-1262 mcg 66% Vitamin B12 (cyanocobalamin) 6 mcg 100% Biotin 40 mcg 13% Zinc (zinc gluconate) 5 mg 33% Copper (cupric oxide) 1 mg 50% Fluoride (sodium fluoride) 0.25 mg † - Other Ingredients
- Description
- Directions
- Tamper Evident Feature
- WARNING
-
CAUTION
Do not use this product if you are allergic to any of the ingredients. Take this product at least 2 hours before or after taking any products containing calcium (including milk, yogurt, other dairy products) or aluminum/magnesium hydroxide (e.g., certain antacids/laxatives). Prolonged daily ingestion of excessive fluoride may result in varying degrees of dental fluorosis. Account for all daily sources of fluoride intake.
-
OTHER INFORMATION
You may report serious side effects to 1-844-640-7607. Store in a cool, dry place at room temperature 20-25° Celsius (68-77° Fahrenheit) away from heat and sunlight. Store in original container.
The numeric identifier on this product's labeling is an assigned product code for use with pharmacy-level, health insurance and state level reimbursement programs and is not intended to denote registration with the FDA.
The manufacturer of this product requires that it be dispensed only under the order of a physician or licensed medical practitioner.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
0.25 mg* FLUORIDE
MULTIVITAMIN TABLETSBonGeo™
PHARMACEUTICALS52796-173-90
Floriva™
.25 mg TABLETS
Chewable Fluoride MultivitaminQuatrefolic®
Patent Ref.: U.S. Patent No. 7,947,662;
Other patents pendingNatural Berry Flavor
Rx Dietary Supplement
90 Chewable Tablets*contains 0.25 mg fluoride
from .55 mg sodium fluorideSUGAR FREE AND GLUTEN FREE
NO ARTIFICIAL COLORS OR DYES
-
INGREDIENTS AND APPEARANCE
FLORIVA
vitamin a acetate, .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol, thiamine, riboflavin, niacinamide, pyridoxine, levomefolate glucosamine, folic acid, cyanocobalamin, biotin, zinc gluconate, cupric oxide, and sodium fluoride tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52796-173 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1000 [iU] BETA CAROTENE (UNII: 01YAE03M7J) (BETA CAROTENE - UNII:01YAE03M7J) BETA CAROTENE 1000 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 75 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 600 [iU] .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 20 [iU] THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.3 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.5 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 15 mg PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 1.8 mg LEVOMEFOLATE GLUCOSAMINE (UNII: Q65PL71Q1A) (LEVOMEFOLATE GLUCOSAMINE - UNII:Q65PL71Q1A) LEVOMEFOLATE GLUCOSAMINE 162 ug FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 100 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 6 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 40 ug ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 5 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1 mg SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCRALOSE (UNII: 96K6UQ3ZD4) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color WHITE (Off white, speckled) Score 2 pieces Shape HEXAGON (6 SIDED) Size 13mm Flavor BERRY Imprint Code 173 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52796-173-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/10/2015 2 NDC:52796-173-12 12 in 1 CARTON 09/10/2015 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 09/10/2015 Labeler - BonGeo Pharmaceuticals, Inc. (964822022)