Label: UPCARD-CA1 TORSEMIDE- torsemide solution
- NDC Code(s): 17030-020-32, 17030-020-96
- Packager: Vetoquinol USA, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated May 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
UpCard-CA1 (torsemide oral solution) is a loop diuretic of the pyridyl sulfonylurea class. Loop diuretics mainly inhibit the Na+/2Cl-/K+ carrier in the ascending limb of the loop of Henle. UpCard-CA1 is an oral solution of 0.2% w/v torsemide in an aqueous mixture containing tromethamine, hydroxyethyl cellulose, saccharin sodium, and propylene glycol.
Torsemide has the structural formula:
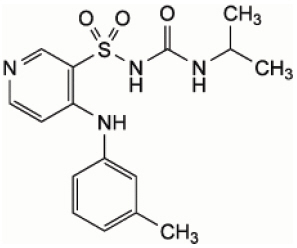
Molecular Formula: C16H20N4O3S
Molecular Weight: 348.42 - Indication:
-
Dosage and Administration:
UpCard-CA1 should be administered orally once daily at a dose of 0.05 to 0.2 mg/lb (0.11 to 0.44 mg/kg) of bodyweight, corresponding to 0.025 to 0.10 mL/lb (0.055 to 0.22 mL/kg).
UpCard-CA1 is intended for long term administration at a dose adapted to the severity of clinical signs of pulmonary edema, results of physical examination, hydration status, and blood urea nitrogen (BUN), serum creatinine, and serum electrolyte levels.
Do not exceed a dose of 0.2 mg/lb (0.44 mg/kg) per day, corresponding to 0.1 mL/lb (0.22 mL/kg).
UpCard-CA1 may be administered with or without food.
UpCard-CA1 Dose Adjustment:
Long term diuretic therapy with UpCard-CA1 should be continued at the lowest effective dose. The dosage should be adjusted to maintain patient comfort, and control clinical signs based on serial physical examination and clinical pathology evaluation performed regularly during the early course of therapy and periodically thereafter (see Precautions).
The dose of UpCard-CA1 may be increased or decreased within the recommended dose range by increments of 25% of the administered dose under veterinary supervision.
-
Contraindications:
Do not administer UpCard-CA1 to dogs with renal failure or anuria. Therapy should be discontinued in dogs with progressive renal disease if increasing azotemia and oliguria occur during the therapy.
Do not administer UpCard-CA1 to dogs with severe dehydration, hypovolemia or hypotension.
Do not administer UpCard-CA1 concomitantly with other loop diuretics (e.g., furosemide).
Do not administer UpCard-CA1 to dogs with hypersensitivity to the active substance, torsemide, or to any of the excipients.
-
Warnings:
User Safety Warnings: Not for use in humans. Keep this and all medications out of the reach of children. Wash hands after use and/or spillage.
In case of accidental human ingestion, seek medical advice immediately and show package insert or the label to the physician. Symptoms of exposure to torsemide may include dryness of the mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, nausea, or vomiting. Additionally, exposure may induce hypovolemia and result in hyperglycemia, hypokalemia, shunt thrombosis, syncope, and ventricular tachycardia.
Animal Safety Warnings:
Keep UpCard-CA1 in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
In case of accidental overdose, provide drinking water and monitor electrolytes. Symptomatic therapy (e.g., fluid therapy) should be provided as medically necessary.
The administration of UpCard-CA1, a loop diuretic, may lead to excessive diuresis which could result in electrolyte imbalance, dehydration, and reduction of plasma volume enhancing the risk of circulatory collapse, thrombosis, and embolism. Dogs receiving UpCard-CA1 should be observed for signs of fluid depletion with electrolyte imbalance.
-
Precautions:
UpCard-CA1 is not indicated for dogs presenting in acute crisis with pulmonary edema, pleural effusion, and/or ascites requiring emergency treatment. The use of injectable diuretic therapy should be considered first in dogs presenting in acute crisis before commencing oral therapy with UpCard-CA1.
UpCard-CA1 is for use only in stable dogs with congestive heart failure caused by MMVD. A diagnosis of MMVD should be made by means of a comprehensive physical and cardiac examination.
Pre-existing electrolyte abnormalities and/or dehydration should be corrected prior to therapy with UpCard-CA1.
The safe administration of UpCard-CA1 relies on regular assessment of treated dogs for the clinical signs of pulmonary edema and potential treatment-related adverse events. Physical examination, hydration status, BUN, serum creatinine, and serum electrolytes should be assessed prior to the initiation of therapy or dose adjustment, and at 24 hours and 48 hours after the start of therapy or dose adjustment. These parameters should be monitored on a monthly basis until they are stable.
Carefully monitor the electrolyte status in cases of concomitant use with products affecting electrolyte balance (e.g., corticosteroids, amphotericin B, and cardiac glycosides).
Concurrent use of drugs that increase the risk of renal injury or renal insufficiency should be avoided.
Concurrent use of non-steroidal anti-inflammatory drugs (NSAIDs) with UpCard-CA1 may result in a decreased natriuretic response and renal impairment.
Concurrent use of aminoglycosides or cephalosporins with UpCard-CA1 may increase the risk of nephrotoxicity and ototoxicity.
The safety of UpCard-CA1 has not been evaluated in dogs with heart failure caused by etiologies other than MMVD. The safe use of UpCard-CA1 has not been evaluated in dogs with congenital heart defects.
The safe use of UpCard-CA1 has not been evaluated in dogs with diabetes mellitus or other serious metabolic diseases.
The safe use of UpCard-CA1 has not been evaluated in dogs used for breeding, or pregnant or lactating bitches.
The dose of UpCard-CA1 may need to be adjusted when administering UpCard-CA1 with digoxin.
UpCard-CA1 may potentially induce an allergic reaction in dogs with sulfonamide allergy.
UpCard-CA1 can reduce renal excretion of salicylates, leading to an increased risk of aspirin toxicity.
-
Adverse Reactions:
In a multi-site European clinical field study, 251 client-owned dogs suffering from edema secondary to congestive heart failure were treated with at least one dose of a tablet formulation of torsemide (n=126) or furosemide (n=125) for a 3-month treatment period. A greater overall frequency of adverse reactions was recorded in the torsemide group (n=184 events) compared with furosemide treated dogs (n=104 events).
The most common adverse reactions associated with torsemide administration involved the urinary system, including polyuria and polydipsia, renal insufficiency, increased BUN and serum creatinine, and urinary incontinence. These findings were noted at greater frequency in torsemide-treated dogs than in the furosemide treatment group.
A relative increase in the risk of serious adverse events due to renal insufficiency (including increased BUN, increased serum creatinine, and renal failure) was observed among torsemide-treated dogs compared with furosemide-treated dogs. Median BUN and serum creatinine levels were greater across all time points in torsemide-treated dogs, and were still high in this group on day 84.
Electrolyte disturbances, including hypokalemia, hypochloremia, hypercalcemia, and hypomagnesemia, were also associated with torsemide therapy. Diarrhea, vomiting, inappetence, and lethargy were also noted in torsemide-treated dogs.
Clinical findings associated with the worsening of congestive heart failure were noted in torsemide-treated dogs, including cough, dyspnea, pulmonary edema, and cardiac arrest.
A total of 30 dogs died during the study, 12 in the torsemide group and 18 in the furosemide group. Euthanasia was the most common cause of death with similar frequency between the treatment groups, and was due to progression of renal failure, deterioration of condition, acute pulmonary edema, acute cardiac death, accidental death, death from another disease condition, or unknown cause.
- Contact Information:
-
Clinical Pharmacology:
Mode of Action: Torsemide is secreted into the tubule lumen via the probenecid sensitive organic acid transport system. The main site of action is the medullary portion of the ascending limb of the loop of Henle. Loop diuretics mainly inhibit the Na+/2Cl-/K+ carrier from the luminal side of the cell.
Inhibition of sodium and chloride ion reabsorption not only results in saluresis but also a decrease in interstitial osmolarity within the renal medulla. This in turn decreases free water reabsorption resulting in increased water excretion/urine production.
Pharmacokinetics: In dogs, after a single intravenous dose at 0.1 mg/kg, the total body clearance was 0.017 L/h∙kg, the volume of distribution was 0.14 L/kg and the terminal half-life was 7.0 hours. The pharmacokinetic parameters following a single dose of the oral solution at 0.1 mg/kg, are presented in Table 1. Torsemide is highly bound to plasma proteins (approximately 98%). A large proportion of the dose (between 61% and 70%) is excreted in the urine as unchanged parent drug. Two metabolites (a dealkylated and a hydroxylated metabolite) were also identified in urine. For the oral solution, dose proportionality was established within the dose range of 0.4 to 0.8 mg/kg. After 14 repeated daily administrations, accumulation was minimal (geometric mean accumulation ratio = 1.11) with steady state being reached after the second administration.
Feeding significantly increased torsemide area under the curve from the time of dosing to the last quantifiable concentration (AUC) by 24% on average but no significant impact on Cmax was detected. When 2 dogs were fed wet food, Tmax was delayed (i.e., 4 h for wet food vs 0.9 h for dry food or fasted).
Table 1. Mean plasma pharmacokinetics for torsemide after a single administration of UpCard-CA1 Parameter Torsemide (mean ± SD) Cmax = maximum plasma concentration Tmax = time to maximum plasma concentration AUC0-inf = area under the plasma concentration time curve from time 0 extrapolated to infinity t1/2 = apparent terminal elimination half life Cmax (µg/mL) 1.21 ± 0.18 Tmax (h) 0.75 ± 0.26 AUC0-inf (µg∙h/mL) 7.50 ± 1.62 t1/2 (h) 7.83 ± 1.58 Bioavailability 96% -
Reasonable Expectation of Effectiveness:
A reasonable expectation of effectiveness may be demonstrated based on evidence such as, but not limited to, pilot data in the target species or studies from published literature.
UpCard-CA1 is conditionally approved pending a full demonstration of effectiveness.
Additional information for Conditional Approvals can be found at www.fda.gov/animalca.
Reasonable expectation of effectiveness for the management of pulmonary edema related to congestive heart failure in dogs with MMVD is based on the results from a randomized multi-site European field safety and effectiveness study using torsemide tablets. Of a total of 251 dogs that were enrolled in the study, 61 dogs with MMVD were considered appropriate for evaluation of the primary endpoint to support reasonable expectation of effectiveness because they were clinically stable and had received furosemide for at least 10 days prior to enrollment. After enrollment, 25 of these dogs were transitioned to torsemide tablets, and 36 dogs continued to be treated with furosemide.
The primary endpoint was response rate at Day 84; a dog was considered to have a successful response to treatment if pulmonary edema and/or pleural effusion or ascites had not increased compared to Day 0. The success rates were 96.0% (24/25) for the torsemide group and 80.6% (29/36) for the furosemide group.
A pharmacokinetic study compared torsemide plasma exposure and urine excretion after a single administration of torsemide tablets and UpCard-CA1 (torsemide oral solution) at 0.1 mg/kg. The relative bioavailability in plasma and urine of UpCard-CA1 were high, although the plasma Cmax and AUC values reached after administration of UpCard-CA1 were slightly lower compared to the tablets. Therefore, similar diuretic activity and safety profiles are expected following administration of each formulation.
-
Target Animal Safety:
In a laboratory safety study, 32 healthy, 6-month-old beagle dogs (16 males and 16 females) were randomly assigned to a placebo control group or were dosed orally once daily for 6 months with UpCard-CA1 at doses of 0.25×, 1×, and 1.5× the maximum daily recommended therapeutic dose (0.11, 0.44, 0.66 mg/kg/day, respectively).
UpCard-CA1 administration resulted in decreased food consumption and dose dependent lower body weight compared to the control group, with the 0.25×, 1×, and 1.5× torsemide groups showing a 4.6%, 9.4% and 11.8% reduction in body weight, respectively. In the 1× and 1.5× torsemide groups, increased urinary output and decreased urine specific gravity were observed. Occasional dehydration and reduced activity/lethargy were also observed in some animals.
UpCard-CA1 administration at 1× and 1.5× resulted in clinical pathology changes consistent with the expected effects of a loop diuretic: erythroid changes consisted of increased red cell mass parameters (i.e., red blood cell count, hemoglobin, and hematocrit) and serum chemistry changes consisted of increased albumin, BUN, and creatinine, and decreased chloride and potassium. These changes are consistent with dehydration secondary to diuresis and the effect of torsemide on the kidneys. UpCard-CA1 treatment had no observed effects on ophthalmologic examination findings, electrocardiography, blood pressure, and body temperature. Concentrations of torsemide increased in an approximate dose proportional manner between 0.11 and 0.66 mg/kg. At the 1× dosage, accumulation was minimal with steady state being reached by, or before, the fourth week of dosing.
Supporting Safety Study: In a second laboratory safety study, 32 healthy, 4 to 5-month-old beagle dogs (16 males and 16 females) were randomly assigned to a placebo control group or were dosed orally once daily for 13 weeks with torsemide tablets at doses of 0.23×, 0.68×, and 1.36× the maximum recommended therapeutic dose (0.1, 0.3 and 0.6 mg/kg, respectively). Water consumption increased in the group administered 0.6 mg/kg compared to the control group. Clinical observations attributed to product administration included erythema of the inner pinnae, the frequency of which increased in a dose-dependent manner.
Changes in clinical pathology were observed as follows: increased hematocrit, BUN, creatinine, and albumin concentrations; decreased plasma potassium, chloride, phosphate, and magnesium concentrations; and increased serum aldosterone levels. There were increases in urine volume, which were accompanied by reductions in specific gravity and urine concentrations of creatinine, sodium, potassium, chloride, and phosphate, and increases in the fractional excretion of calcium and phosphate. These changes appeared to be dose dependent. On necropsy, kidney weights were significantly higher in the 0.6 mg/kg group compared to the control group. Histopathological changes to the renal cortex and medulla were minimal to mild. Systemic exposure (AUClast) increased in a dose proportional manner between 0.1 and 0.6 mg/kg. Accumulation was minimal with steady state being reached by, or before, the fourth week of dosing.
- Storage:
- How Supplied:
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 32 mL Bottle Label
NDC 17030-020-32
UpCard®-CA1
(torsemide oral solution)
2 mg/mL
Diuretic for oral use in dogs only.Conditionally approved by FDA pending
a full demonstration of effectiveness
under application number 141-577.It is a violation of Federal law to use this
product other than as directed in the labeling.CAUTION: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.Net Contents: 32 mL
vetoquinol

-
INGREDIENTS AND APPEARANCE
UPCARD-CA1 TORSEMIDE
torsemide solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17030-020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TORSEMIDE (UNII: W31X2H97FB) (TORSEMIDE - UNII:W31X2H97FB) TORSEMIDE 2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17030-020-32 1 in 1 CARTON 1 32 mL in 1 BOTTLE, PLASTIC 2 NDC:17030-020-96 1 in 1 CARTON 2 96 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141577 05/13/2024 Labeler - Vetoquinol USA, Inc. (106824209) Establishment Name Address ID/FEI Business Operations Vetoquinol N.-A. INC 202919940 ANALYSIS, LABEL, MANUFACTURE, PACK

