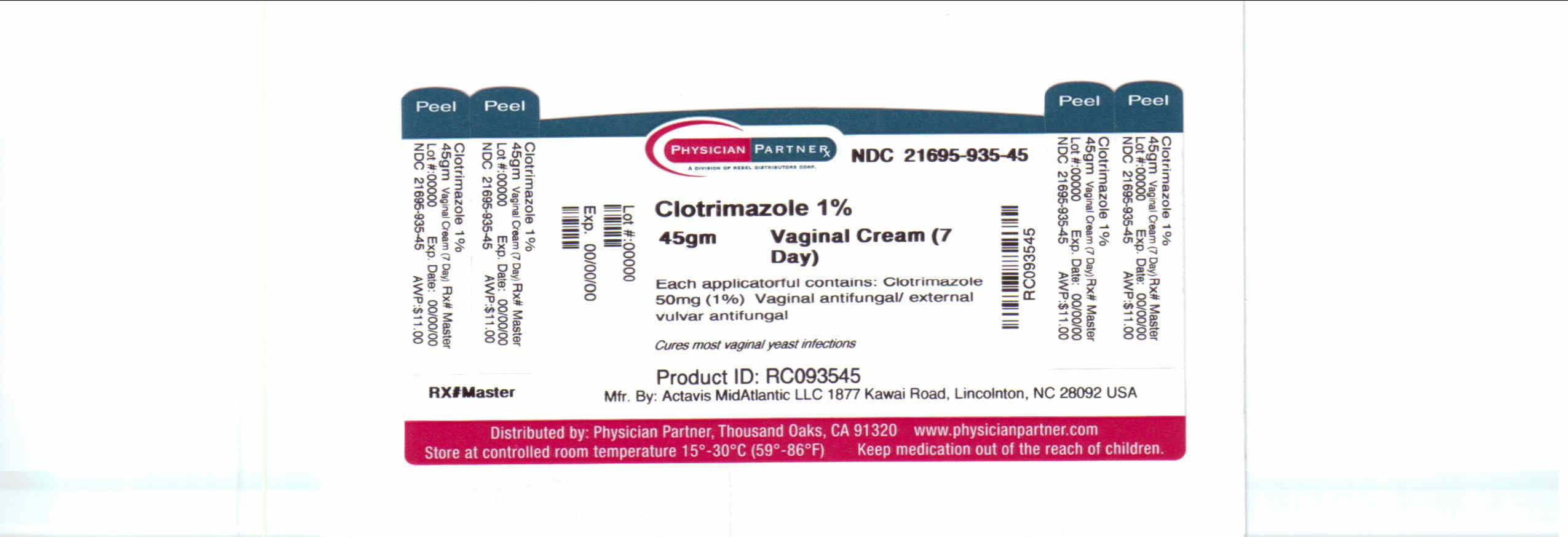
Label: CLOTRIMAZOLE cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-935-45 - Packager: Rebel Distributors Corp.
- This is a repackaged label.
- Source NDC Code(s): 0472-0220
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 11, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
For vaginal or external vulvar use only.
Do not use:
° If you have abdominal pain, fever, or a foul smelling vaginal discharge.
Contact your doctor immediately.
° In children less than 12 years of age.
Ask a doctor before use if you have:
° vaginal or vulvar itching and discomfort for the first time. If you have had a doctor diagnose a vaginal yeast infection before and have the same symptoms now, use this cream as directed for 7 days in a row.
° symptoms that return within 2 months or infections that do not clear up easily with proper treatment. You could be pregnant, or there could be a serious underlying medical cause for your infections, including diabetes or a damaged immune system (including damage from infection from HIV - the virus that causes AIDS). Please read enclosed educational pamphlet.
When using this product do not use tampons.
Stop use and ask a doctor if:
symptoms do not get better in 3 days or infection isn't gone in 7 days. You may have a condition other than a yeast infection.
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
° before using this product, read the enclosed pamphlet
° fill the applicator and insert one applicatorful of cream into the vagina, preferably at bedtime. Repeat this procedure 7 days in a row. Throw applicator away after use.
° for relief of external vulvar itching, squeeze a small amount of cream onto your finger and gently spread the cream onto the irritated area of the vulva. Use once or twice a day for up to 7 days as needed to relieve external vulvar itching. The cream should not be used for vulvar itching due to causes other than a yeast infection.
-
Other Information
° store at room temperature between 2° and 30°C (36° to 86°F)°
° see end flaps of carton and tube for lot number and expiration date
° safety sealed: the tube opening should be sealed. If seal has been punctured or is not visible, do not use the product.
Questions? 1-800-432-8534 (select option #2) between 9 am and 4 pm EST, Monday-Friday.
- Inactive ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CLOTRIMAZOLE
clotrimazole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21695-935(NDC:0472-0220) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOTRIMAZOLE (UNII: G07GZ97H65) (CLOTRIMAZOLE - UNII:G07GZ97H65) CLOTRIMAZOLE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL ESTERS WAX (UNII: D072FFP9GU) OCTYLDODECYL STEARATE (UNII: K6F16QGO28) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-935-45 45 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074165 07/16/1993 Labeler - Rebel Distributors Corp. (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp. 118802834 RELABEL, REPACK