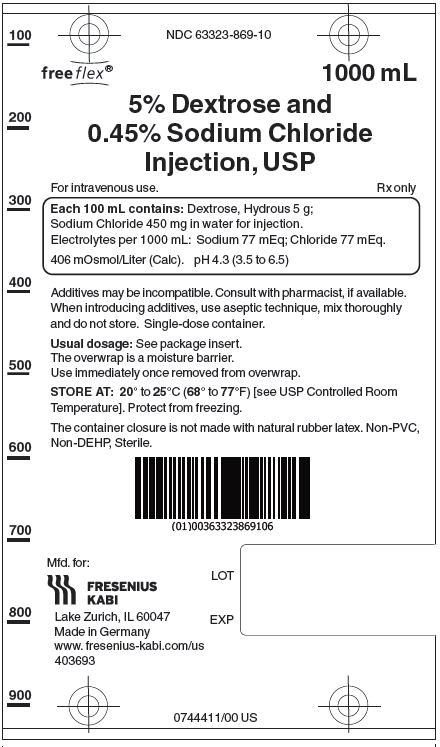
Label: DEXTROSE AND SODIUM CHLORIDE- dextrose monohydrate and sodium chloride injection, solution
- NDC Code(s): 63323-869-10, 63323-869-74, 63323-869-75
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 14, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
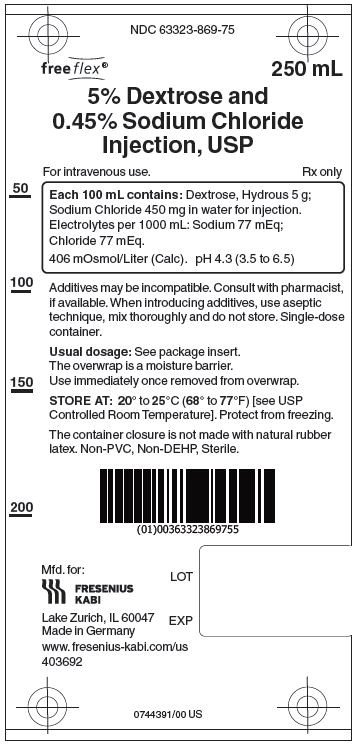
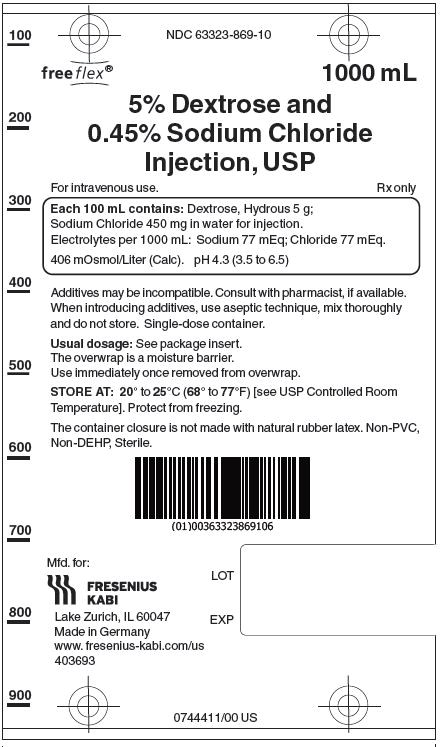
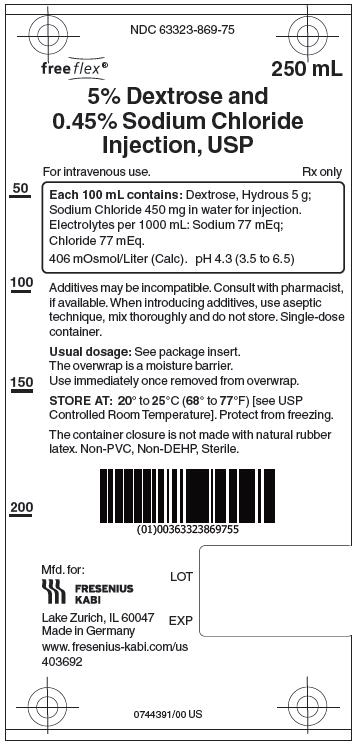
5% Dextrose and 0.45% Sodium Chloride Injection, USP solutions are sterile and nonpyrogenic. They are large volume parenteral solutions containing 5 grams per 100 mL of Dextrose monohydrate and 0.45 grams per 100 mL of Sodium Chloride in water for injection intended for intravenous administration.
Each 100 mL of 5% Dextrose and 0.45% Sodium Chloride Injection, USP contains dextrose, hydrous 5g and sodium chloride 0.45 g in water for injection. Electrolytes per 1000 mL: sodium (Na+), 77 mEq; chloride (Cl-) 77 mEq. The osmolarity is 406 mOsmol/L (calc.), which is hypertonic. The caloric value is 170 kcal/L. The pH is 4.3 (3.5 to 6.5).
5% Dextrose and 0.45% Sodium Chloride Injection, USP contains no bacteriostat, antimicrobial agent or added buffer and is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
5% Dextrose and 0.45% Sodium Chloride Injection, USP is a parenteral fluid, nutrient and electrolyte replenisher.
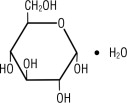
Dextrose, USP is chemically designated D-glucose monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Water for Injection, USP is chemically designated H2O.
The flexible container is fabricated from a specially formulated non-plasticized, film containing polypropylene and thermoplastic elastomers (freeflex® bag). The amount of water that can permeate from the container into the overwrap is insufficient to affect the solution significantly.
Solutions in contact with the flexible container can leach out certain of the container's chemical components in very small amounts within the expiration period. The suitability of the container material has been confirmed by tests in animals according to USP biological tests for plastic containers.
-
CLINICAL PHARMACOLOGY
When administered intravenously, these solutions provide a source of water, carbohydrate and electrolytes.
Solutions which provide combinations of hypotonic or isotonic concentrations of dextrose and of sodium chloride are suitable for parenteral maintenance or replacement of water and electrolyte requirements with minimal carbohydrate calories.
Solutions containing carbohydrate in the form of dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl¯) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl¯) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) and chloride (Cl¯) are largely under the control of the kidney which maintains a balance between intake and output.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirements range from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production). Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
Excessive administration of potassium-free solutions may result in significant hypokalemia.
In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
-
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions to patients receiving corticosteroids or corticotropin.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Pregnancy. Animal reproduction studies have not been conducted with dextrose or sodium chloride. It is also not known whether dextrose or sodium chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose or sodium chloride should be given to a pregnant woman only if clearly needed.
Pediatric Use. The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants, the volume of fluid may affect fluid and electrolyte balance.
Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
Geriatric Use. An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Sodium ions are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
In the event of overhydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.
-
DOSAGE AND ADMINISTRATION
The dose is dependent upon the age, weight and clinical condition of the patient.
As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
Drug Interactions
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
INSTRUCTIONS FOR USE:
Check flexible container solution composition, lot number, and expiry date.
Do not remove solution container from its overwrap until immediately before use. Use sterile equipment and aseptic technique.
To Open
- Turn solution container over so that the text is face down. Using the pre-cut corner tabs, peel open the overwrap and remove solution container.
- Check the solution container for leaks by squeezing firmly. If leaks are found, or if the seal is not intact, discard the solution.
- Do not use if the solution is cloudy or a precipitate is present.
To Add Medication
- Identify WHITE Additive Port with arrow pointing toward container.
- Immediately before injecting additives, break off WHITE Additive Port Cap with the arrow pointing toward container.
- Hold base of WHITE Additive Port horizontally.
- Insert needle horizontally through the center of WHITE Additive Port's septum and inject additives.
- Mix container contents thoroughly.
Preparation for Administration
- Immediately before inserting the infusion set, break off BLUE Infusion Port Cap with the arrow pointing away from container.
- Use a non-vented infusion set or close the air-inlet on a vented set.
- Close the roller clamp of the infusion set.
- Hold the base of BLUE Infusion Port.
- Insert spike through BLUE Infusion Port by rotating wrist slightly until the spike is inserted.
NOTE: See full directions accompanying administration set.
WARNING: Do not use flexible container in series connections.
-
HOW SUPPLIED
5% Dextrose and 0.45 % Sodium Chloride Injection, USP is supplied in single-dose flexible plastic containers in 250 mL, 500 mL and 1000 mL sizes as follows:
Product Name Size Number per Carton NDC Product Code 5% Dextrose and 0.45%
Sodium Chloride
Injection, USP250 mL 30 NDC 63323-869-75 869175 5% Dextrose and 0.45%
Sodium Chloride
Injection, USP500 mL 20 NDC 63323-869-74 869174 5% Dextrose and 0.45%
Sodium Chloride
Injection, USP1000 mL 10 NDC 63323-869-10 869110 The container closure is not made with natural rubber latex. Non-PVC, Non-DEHP, Sterile. Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat.
Protect from freezing. Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Manufactured for:
Lake Zurich, IL 60047
Made in Germany
451684
www.fresenius-kabi.com/usIssued: September 2020
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEXTROSE AND SODIUM CHLORIDE
dextrose monohydrate and sodium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63323-869 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.45 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63323-869-75 30 in 1 CASE 10/26/2020 1 250 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:63323-869-74 20 in 1 CASE 10/26/2020 2 500 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC:63323-869-10 10 in 1 CASE 10/26/2020 3 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211276 10/26/2020 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Deutschland GmbH 506719546 ANALYSIS(63323-869) , MANUFACTURE(63323-869)