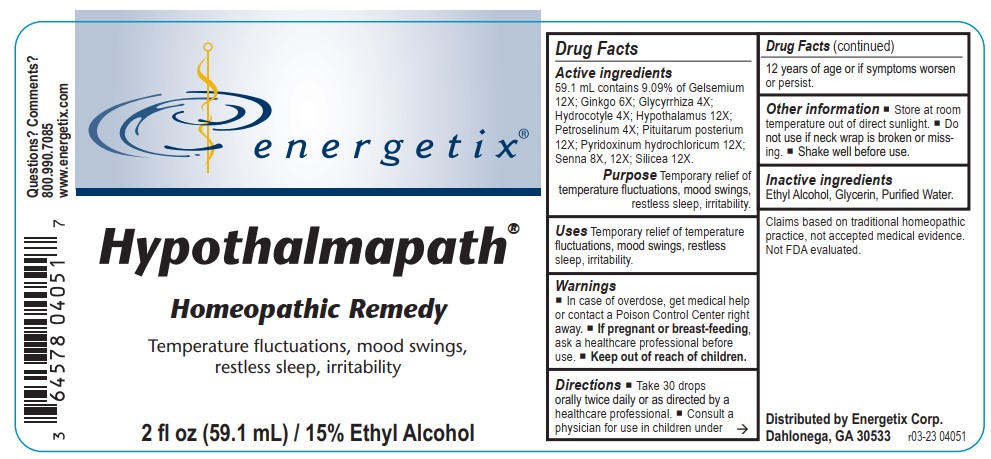
Label: HYPOTHALMAPATH- gelsemium, ginkgo, glycyrrhiza, hydrocotyle, hypothalamus, petroselinum, pituitarum posterium, pyridoxinum hydrochloricum, senna, silicea liquid
- NDC Code(s): 64578-0171-1
- Packager: Energetix Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients 59.1 mL contains 9.09% of Gelsemium 12X; Ginkgo 6X; Glycyrrhiza 4X; Hydrocotyle 4X; Hypothalamus 12X; Petroselinum 4X; Pituitarum posterium 12X; Pyridoxinum hydrochloricum 12X; Senna 8X, 12X; Silicea 12X.
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYPOTHALMAPATH
gelsemium, ginkgo, glycyrrhiza, hydrocotyle, hypothalamus, petroselinum, pituitarum posterium, pyridoxinum hydrochloricum, senna, silicea liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64578-0171 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 12 [hp_X] in 59.1 mL GINKGO (UNII: 19FUJ2C58T) (GINKGO - UNII:19FUJ2C58T) GINKGO 6 [hp_X] in 59.1 mL LICORICE (UNII: 61ZBX54883) (LICORICE - UNII:61ZBX54883) LICORICE 4 [hp_X] in 59.1 mL CENTELLA ASIATICA WHOLE (UNII: 7M867G6T1U) (CENTELLA ASIATICA - UNII:7M867G6T1U) CENTELLA ASIATICA WHOLE 4 [hp_X] in 59.1 mL BOS TAURUS HYPOTHALAMUS (UNII: S6G2NLH4Y7) (BOS TAURUS HYPOTHALAMUS - UNII:S6G2NLH4Y7) BOS TAURUS HYPOTHALAMUS 12 [hp_X] in 59.1 mL PETROSELINUM CRISPUM WHOLE (UNII: 1WZA4Y92EX) (PETROSELINUM CRISPUM - UNII:1WZA4Y92EX) PETROSELINUM CRISPUM WHOLE 4 [hp_X] in 59.1 mL SUS SCROFA PITUITARY GLAND, POSTERIOR (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND, POSTERIOR - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND, POSTERIOR 12 [hp_X] in 59.1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 12 [hp_X] in 59.1 mL SENNA LEAF (UNII: AK7JF626KX) (SENNA LEAF - UNII:AK7JF626KX) SENNA LEAF 8 [hp_X] in 59.1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 59.1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64578-0171-1 59.1 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/03/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/03/2017 Labeler - Energetix Corporation (969572502)