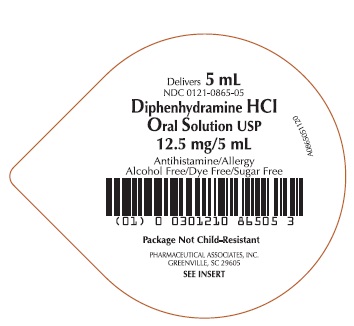
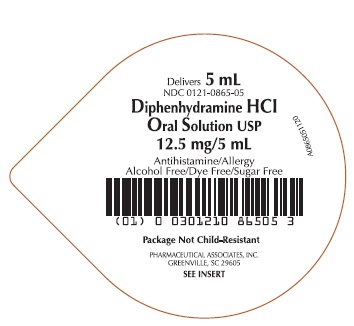
Label: DIPHENHYDRAMINE HCL- diphenhydramine hydrochloride solution
-
NDC Code(s):
0121-0865-00,
0121-0865-05,
0121-0865-30,
0121-1730-00, view more0121-1730-10, 0121-1730-30
- Packager: PAI Holdings, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
- Warnings
-
Directions
- find right dose on chart below
- mL = milliliter
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 doses in 24 hours
Age (yr) Dose (mL) children under 2 years do not use children 2 to 5 years do not use unless directed by a doctor children 6 to 11 years 5 mL to 10 mL Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HCL
diphenhydramine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0121-0865 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white (CLEAR) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-0865-00 10 in 1 CASE 06/18/2020 1 10 in 1 TRAY 1 NDC:0121-0865-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:0121-0865-30 3 in 1 CASE 06/18/2020 2 10 in 1 TRAY 2 NDC:0121-0865-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/18/2020 DIPHENHYDRAMINE HCL
diphenhydramine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0121-1730 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white (CLEAR) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-1730-00 10 in 1 CASE 06/18/2020 1 10 in 1 TRAY 1 NDC:0121-1730-10 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:0121-1730-30 3 in 1 CASE 06/18/2020 2 10 in 1 TRAY 2 NDC:0121-1730-10 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/18/2020 Labeler - PAI Holdings, LLC (044940096) Establishment Name Address ID/FEI Business Operations PAI Holdings, LLC dba Pharmaceutical Associates, Inc. and dba PAI Pharma 097630693 manufacture(0121-0865, 0121-1730)