Label: ASSORTED FRUIT PANNED CHEW- calcium carbonate tablet, chewable
- NDC Code(s): 37808-063-20
- Packager: HEB
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
ASK DOCTOR/PHARMACIST
Ask a doctor or pharmacist before use if you are now taking a prescription drug. Antacids may interact with certain prescription drugs.
Do not take more than 5 chews in a 24-hour period, or use the maximum dosage for more than 2 weeks, except under the advice and supervision of a physician.
When using this product constipation may occur.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive Ingredients: Beeswax, carmine, carnauba wax, citric acid, corn starch, corn syrup, DL-alpha tocopherol, ethyl acetate, FD&C
blue no. 1 aluminum lake, FD&C red no. 40 aluminum lake, FD&C yellow no. 5 lake (tartrazine), FD&C yellow no. 6, FD&C yellow no. 6
aluminum lake, gum arabic, hydrogenated coconut oil, medium chain triglycerides, methyl paraben, modified corn starch, natural and
artificial flavors, pregelatinized corn starch, propylene glycol, propyl paraben, phosphoric acid, purified water, shellac, sodium benzoate, sorbic acid, sorbitol, soy lecithin, soybean oil, sucrose and titanium dioxide.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASSORTED FRUIT PANNED CHEW
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-063 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color orange, yellow, pink Score score with uneven pieces Shape ROUND Size 70mm Flavor ORANGE, LEMON, STRAWBERRY Imprint Code FC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-063-20 120 in 1 BOTTLE; Type 0: Not a Combination Product 07/10/2016 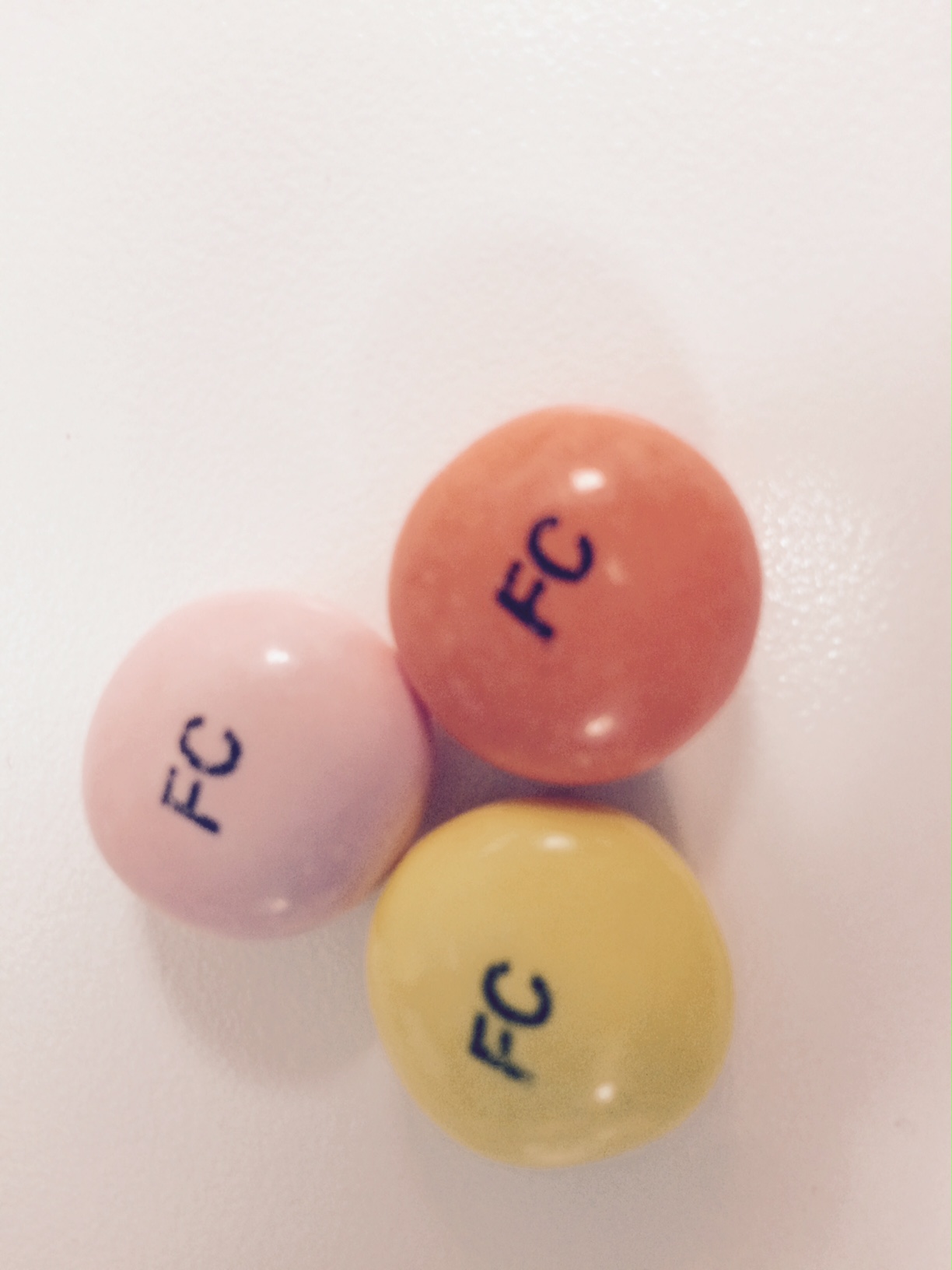
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 07/10/2016 Labeler - HEB (007924756) Registrant - BestCo Inc. (002149136) Establishment Name Address ID/FEI Business Operations BestCo Inc. 002149136 manufacture(37808-063)


