Label: NATUROPATHICA CHLOROPHYLL AND SALICYLIC ACID SPOT TREATMENT- salicylic acid gel
- NDC Code(s): 64657-002-02
- Packager: Naturopathica Holistic Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
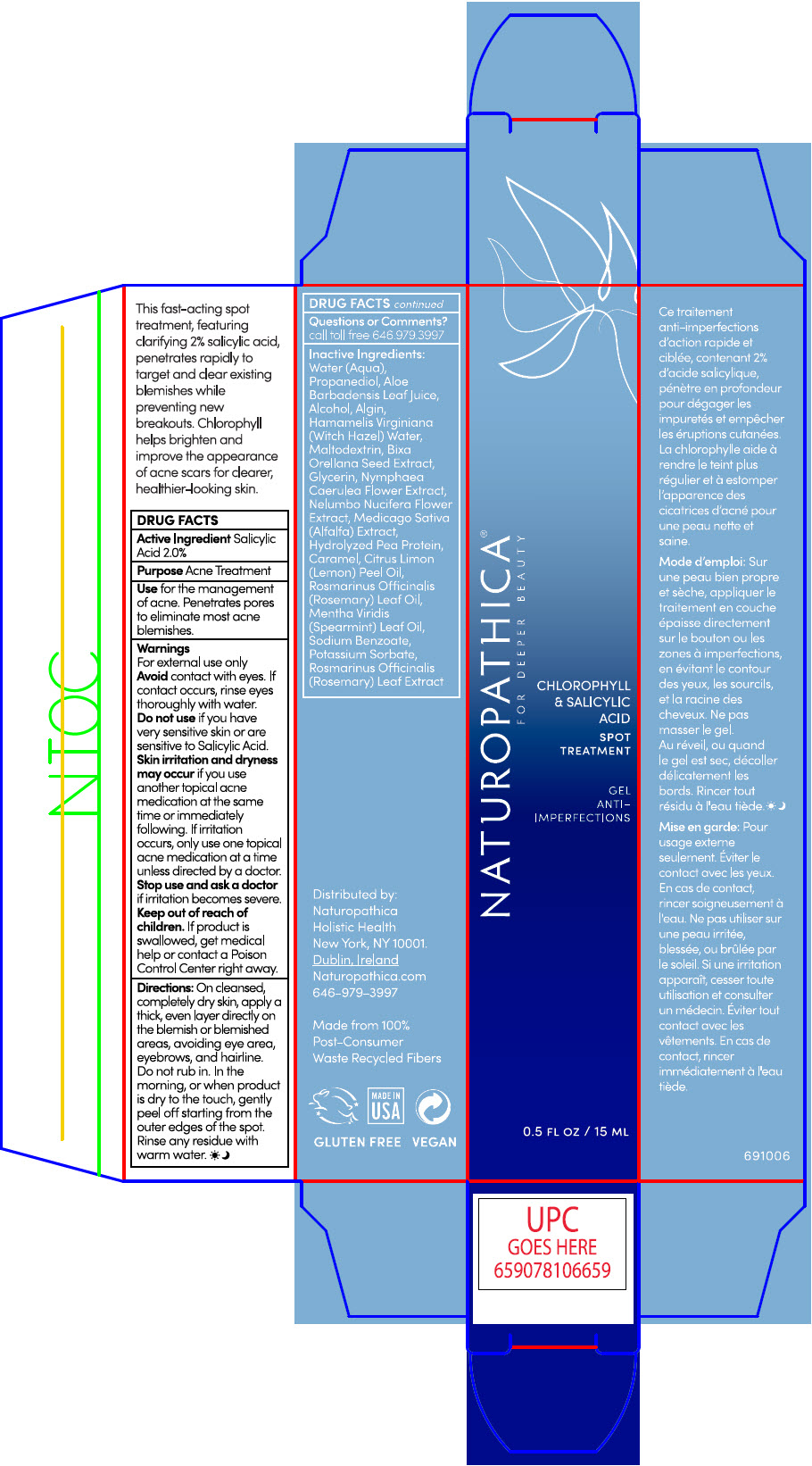
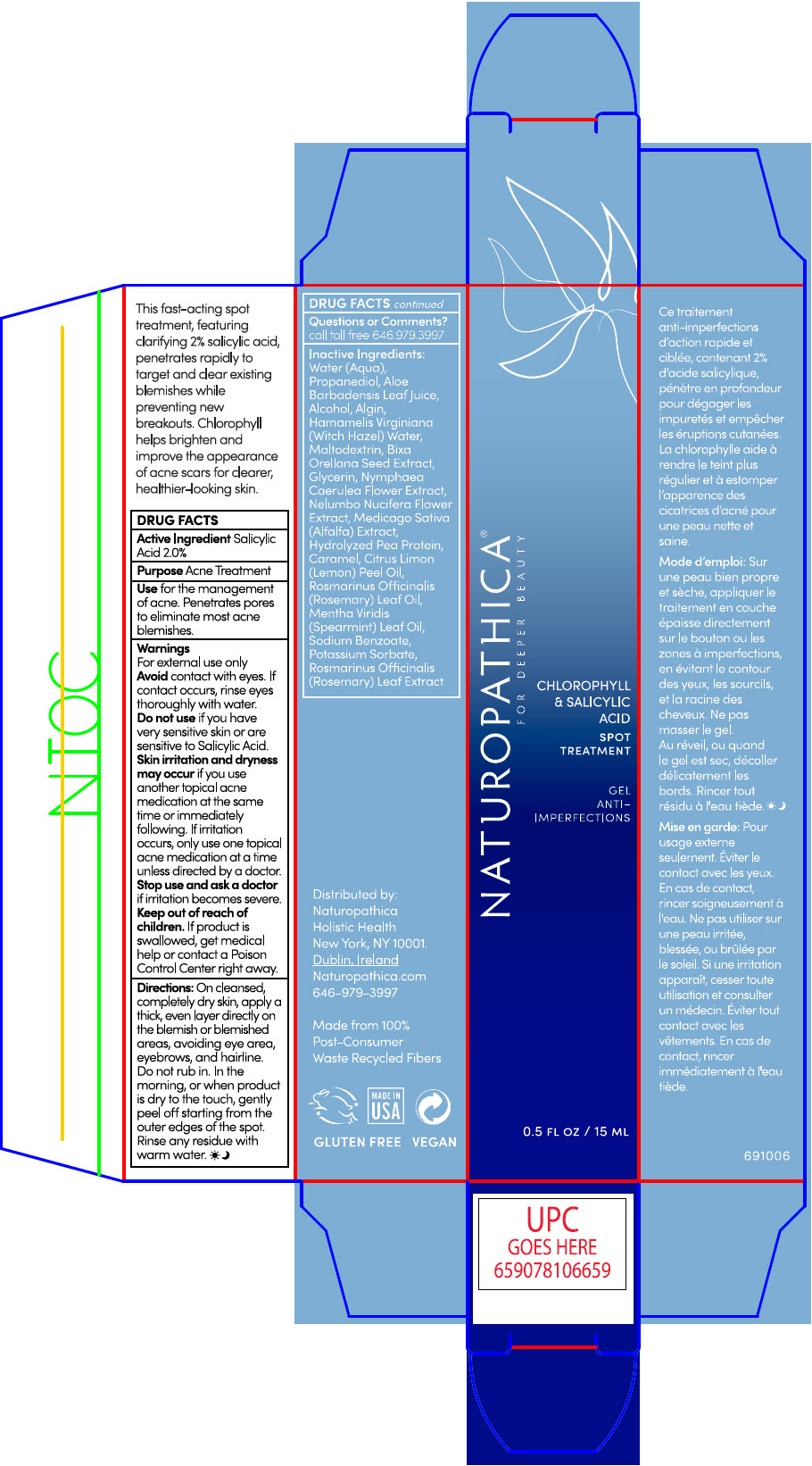
- ACTIVE INGREDIENTS
- PURPOSE
- Use
-
Warnings
For external use only
When using this product
- Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
- Do not use if you have very sensitive skin or are sensitive to Salicylic Acid.
- skin irritation and dryness may occur if you use another topical acne medication at the same time or immediately following. If irritation occurs, only use one topical acne medication at a time unless directed by a doctor.
-
Directions
On cleansed, completely dry skin, apply a thick, even layer directly on the blemish or blemished areas avoiding eye area, eyebrows, and hairline. Do not rub in. IN the morning or when product is dry to the touch, gently peel off starting from the outer edges of the spot. Rinse any residue with warm water.
- Questions or comments?
-
INACTIVE INGREDIENTS
WATER (AQUA), PROPANEDIOL, ALOE BARBADENSIS LEAF JUICE,ALCOHOL, ALGIN, HAMAMELIS VIRGINIANA (WITCH HAZEL) WATER, MALTODEXTRIN, BIXA ORELLANA SEED EXTRACT, GLYCERIN, NYMPHAEA CAERULEA FLOWER EXTRACT, NELUMBO NUCIFERA FLOWER EXTRACT, MEDICAGO SATIVA (ALFALFA) EXTRACT, HYDROLYZED PEA PROTEIN, CARAMEL, CITRUS LIMON (LEMON) PEEL OIL, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF OIL, MENTHA VIRIDIS (SPEARMINT) LEAF OIL, SODIUM BENZOATE, POTASSIUM SORBATE, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT.
CERTIFIED ORGANIC
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 15 ML Tube Carton
-
INGREDIENTS AND APPEARANCE
NATUROPATHICA CHLOROPHYLL AND SALICYLIC ACID SPOT TREATMENT
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64657-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) SODIUM ALGINATE (UNII: C269C4G2ZQ) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) MALTODEXTRIN (UNII: 7CVR7L4A2D) BIXA ORELLANA SEED (UNII: O87354RZ5A) GLYCERIN (UNII: PDC6A3C0OX) NYMPHAEA CAERULEA FLOWER (UNII: S9560USZ74) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) MEDICAGO SATIVA WHOLE (UNII: DJO934BRBD) CARAMEL (UNII: T9D99G2B1R) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) ROSEMARY OIL (UNII: 8LGU7VM393) SPEARMINT OIL (UNII: C3M81465G5) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ROSEMARY (UNII: IJ67X351P9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64657-002-02 1 in 1 CARTON 03/31/2020 1 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/31/2020 Labeler - Naturopathica Holistic Health (104302175)