Label: TEXACLEAR KIDS ALLERGY- chlophedianol hcl pyrilamine maleate liquid
- NDC Code(s): 58809-925-08
- Packager: GM Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
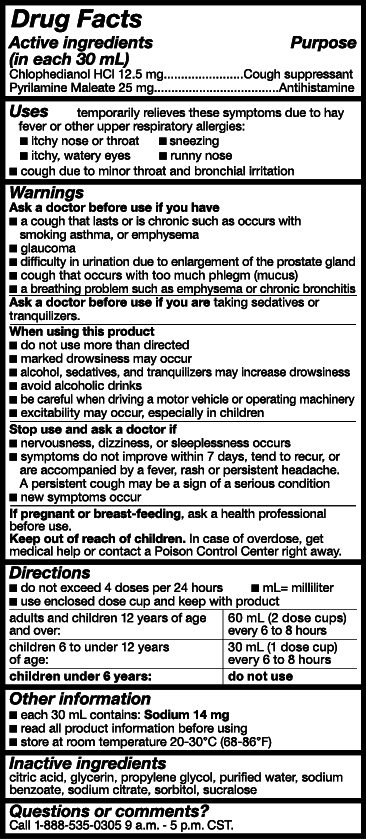
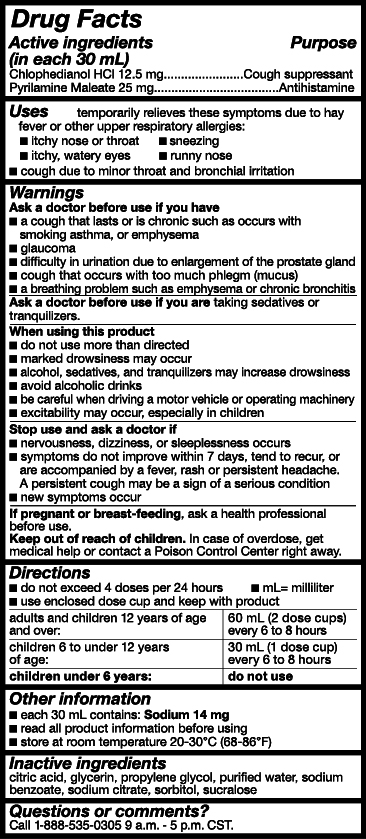
- Active Ingredients
- Purpose
- Uses
- Warnings
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
- Directions
- Other Information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL
TexaClear® Kids Allergy + Cough
NDC 58809-925-08
8 fl. oz. (237 mL)Chlophedianol HCl – Cough Suppressant
Pyrilamine Maleate – AntihistamineTamper evident: do not use if foil seal under cap is broken on missing
- Gluten Free
- Dye Free
- Sugar Free
- Alcohol Free
- Acetaminophen Free
Ages 6+
Dose every 6 to 8 hours- Sneezing
- Runny Nose
- Watery Eyes
- Itchy Nose & Throat
- Cough
Distributed by:
GM Pharmaceuticals, Inc. Fort Worth, TX 76118
-
INGREDIENTS AND APPEARANCE
TEXACLEAR KIDS ALLERGY
chlophedianol hcl pyrilamine maleate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58809-925 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOPHEDIANOL HYDROCHLORIDE (UNII: 69QQ58998Y) (CHLOPHEDIANOL - UNII:42C50P12AP) CHLOPHEDIANOL HYDROCHLORIDE 12.5 mg in 30 mL PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 25 mg in 30 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58809-925-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/15/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M12 06/15/2016 Labeler - GM Pharmaceuticals, Inc. (793000860) Establishment Name Address ID/FEI Business Operations Sovereign Pharmaceuticals, LLC 623168267 manufacture(58809-925)