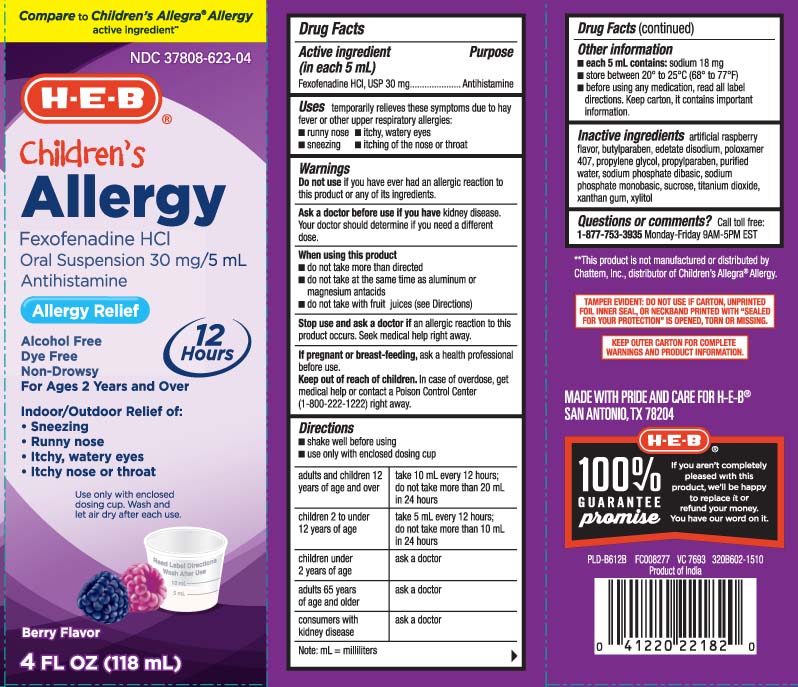
Label: CHILDRENS ALLERGY- fexofenadine hcl suspension
- NDC Code(s): 37808-623-04
- Packager: H E B
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
-
Direction
- shake well before using
- use only with enclosed dosing cup
adults and children 12 years of age and over
take 10 mL every 12 hours: do not take more than 20 mL in 24 hours children 2 to under 12 years of age take 5 mL every 12 hours; do not take more than 10 mL in 24 hours children under 2 years of age ask a doctor adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor
Note: mL = mililiters - Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Compare to Children's Allegra® Allergy active ingredient**
Childrens
ALLERGY
Fexofenadine HCl
Oral Suspension 30 mg/5mL
Antihistamine
Allergy Relief
Alcohol Free
Dye Free
Non-Drowsy
For Ages 2 years and Over
12 HOUR
Indoor/Outdoor Relief of:
- Sneezing
- Runny nose
- Runny nose
- Itchy, watery eyes
- Itchy nose or throat
Berry Flavor
FL OZ (mL)
Use only with enclosed dosing cup. Wash and let air dry after each use.
*This product is not manufactured or distributed by Chattem Inc., distributor of Children's Allegra® Allergy.
TAMPER EVIDENT: DO NOT USE IF CARTON, UNPRINTED FOIL INNER SEAL, OR NECKBAND PRINTED WITH "SEALED FOR YOUR PROTECTION" IS OPENED TORN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
MADE WITH PRIDE AND CARE FOR H-E-B®
SAN ANTONIO, TX 78204
- Product Label
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY
fexofenadine hcl suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-623 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) EDETATE DISODIUM (UNII: 7FLD91C86K) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-623-04 1 in 1 CARTON 04/30/2021 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203330 04/30/2021 Labeler - H E B (007924756)