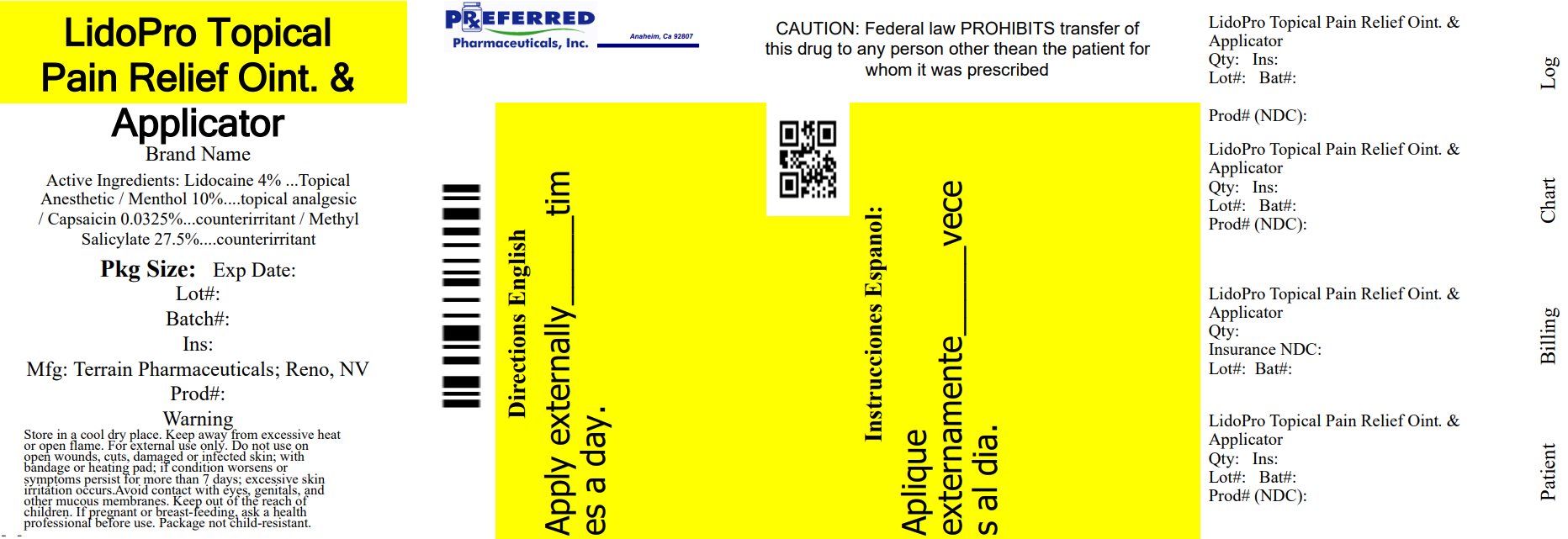
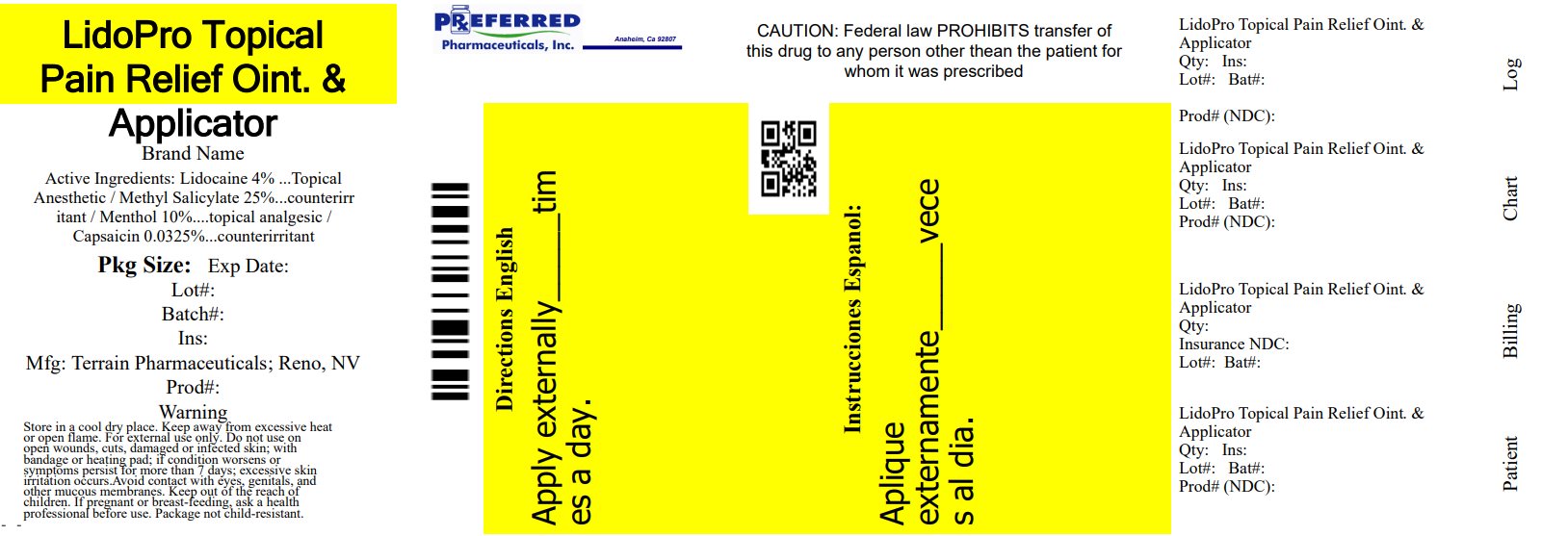
Label: LIDOPRO- capsaicin, lidocaine, menthol, and methyl salicylate ointment
- NDC Code(s): 68788-8314-1
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 53225-1020
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses
-
Warnings
For External Use Only
Do Not Use
- •
- On damaged, irritated, or infected skin
- •
- With a bandage or heating pad
- •
- If you are allergic to any ingredients in this product
Stop Use and Ask a Doctor If:
- •
- Conditions worsens
- •
- Excessive skin irritation develops
- •
- Symptoms persist for more than 7 days, or symptoms clear up and occur again within 3 days
- Directions
- Other Information
-
Inactive Ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Ammonium Acryloyldimethyltaurate/VP Copolymer, Cetyl Alcohol, Chamomilla Recutita Matricaria Flower Extract, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Inulin Lauryl Carbamate, PEG-100 Stearate, Phenoxyethanol, Stearic Acid, Triethanolamine, Water.
- Questions or Comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LIDOPRO
capsaicin, lidocaine, menthol, and methyl salicylate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-8314(NDC:53225-1020) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.000325 g in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.04 g in 1 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 0.1 g in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 0.275 g in 1 g Inactive Ingredients Ingredient Name Strength INULIN (UNII: JOS53KRJ01) STEARIC ACID (UNII: 4ELV7Z65AP) ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CETYL ALCOHOL (UNII: 936JST6JCN) CHAMOMILE (UNII: FGL3685T2X) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-8314-1 92 g in 1 BOTTLE; Type 0: Not a Combination Product 07/08/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 07/08/2022 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-8314)