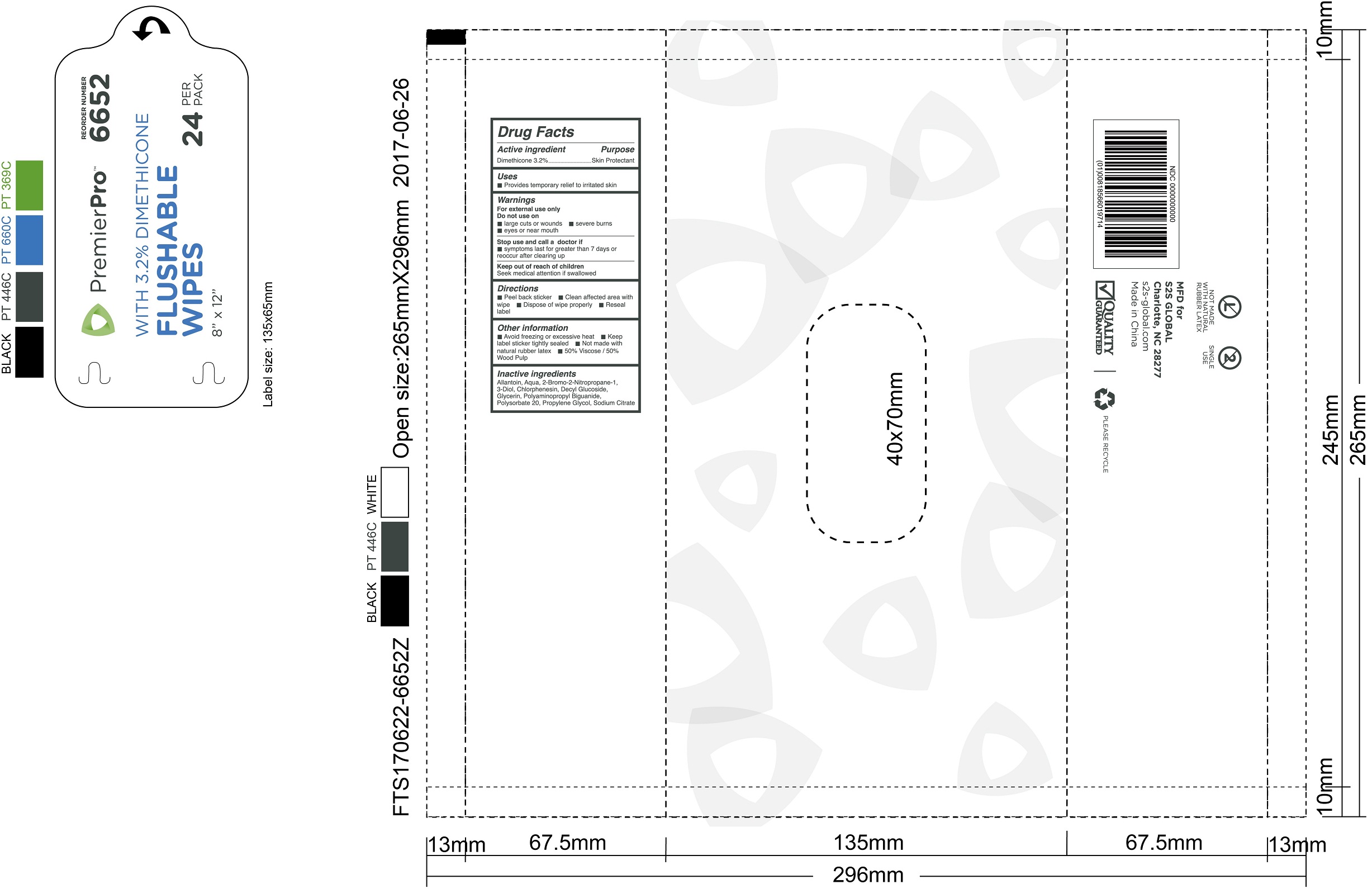
Label: PREMIERPRO FLUSHABLE WIPES WITH DIMETHICONE- dimethicone swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 61312-010-01 - Packager: HANGZHOU GUOGUANG TOURING COMMODITY CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 9, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses:
- Warnings:
-
Directions:
Pull back label and remove wipes
• Thoroughly cleanse soiled area, using one wipe at a time
•Use as often as necessary
•Dispose of cloths in a waste receptacle
•Do not flush
Heating Instructions:
• Wipes may be used at room temperature but if heating is desired, wipes may be warmed in a wipes warmer. Wipes should be warmed:
• in a single warming session at temperatures not exceeding 52°C (125°F) and
• for a maximum of 4 days. Discard wipes that have been:
• Exposed to warming temperatures exceeding 52C (125F); or
• Stored longer than 4 days within the warmer; or
• Been warmed, allowed to cool, and then re-warmed.
- Other Information:
- Inactive Ingredients:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
PREMIERPRO FLUSHABLE WIPES WITH DIMETHICONE
dimethicone swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61312-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 32 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYSORBATE 20 (UNII: 7T1F30V5YH) ALLANTOIN (UNII: 344S277G0Z) CHLORPHENESIN (UNII: I670DAL4SZ) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61312-010-01 24 in 1 CASE 07/13/2017 1 216 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 07/13/2017 Labeler - HANGZHOU GUOGUANG TOURING COMMODITY CO., LTD. (526890634) Establishment Name Address ID/FEI Business Operations HANGZHOU GUOGUANG TOURING COMMODITY CO., LTD. 526890634 manufacture(61312-010)