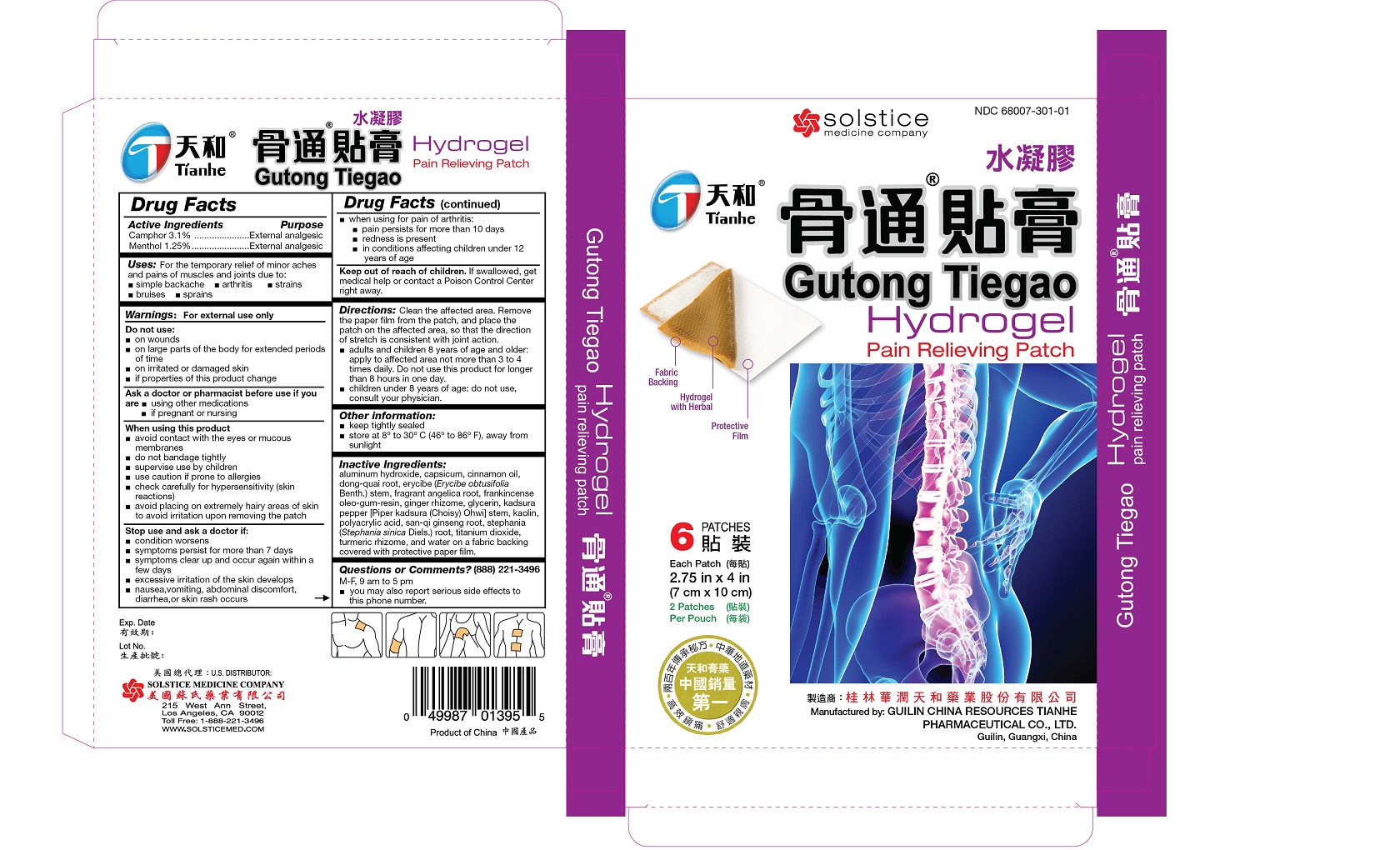
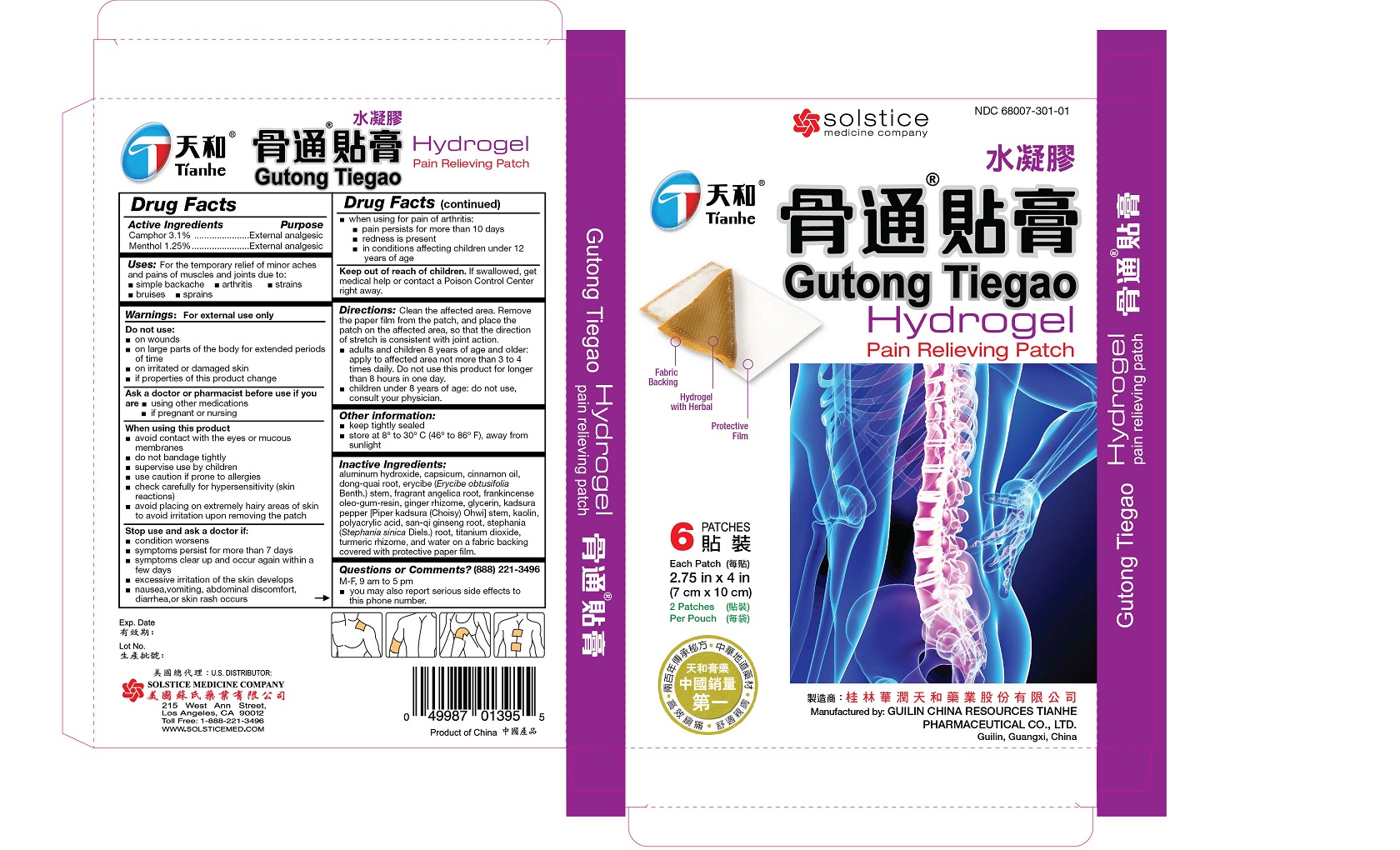
Label: GUTONG TIEGAO PAIN RELIEVING- camphor and menthol patch
- NDC Code(s): 68007-301-01
- Packager: GUILIN CHINA RESOURCES TIANHE PHARMACEUTICAL CO LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 11, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
condition worsens
symptoms persist for more than 7 days
symptoms clear up and occur again within a few days
excessive irritation of the skin develops
nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
when using for pain of arthritis:
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years of age
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Clean the affected area. Remove the paper film from the patch, and place the patch on the affected area, so that the direction of stretch is consistent with joint action.
adults and children 8 years of age and older: apply to affected area not more than 3 to 4 times daily. Do not use this product for longer than 8 hours in one day.
children under 8 years of age: do not use, consult your physician
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
aluminum hydroxide, capsicum, cinnamon oil, dong-quai root, erycibe (Erycibe obtusifolia Benth.) stem, fragrant angelica root, frankincense oleo-gum-resin, ginger rhizome, glycerin, kadsura pepper [Piper kadsura (Choisy) Ohwi] stem, kaolin, polyacrylic acid, san-qi ginseng root, stephania (Stephania sinica Diels.) root, titanium dioxide, turmeric rhizome, and water on a fabric backing covered with protective paper film.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUTONG TIEGAO PAIN RELIEVING
camphor and menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68007-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 248 mg MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 100 mg Inactive Ingredients Ingredient Name Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CAPSICUM (UNII: 00UK7646FG) CINNAMON OIL (UNII: E5GY4I6YCZ) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) ERYCIBE OBTUSIFOLIA STEM (UNII: V1G1S38CQI) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) FRANKINCENSE (UNII: R9XLF1R1WM) ZINGIBER OFFICINALE WHOLE (UNII: IN6Q3S3414) GLYCERIN (UNII: PDC6A3C0OX) PIPER KADSURA STEM (UNII: 80IOP41EL7) KAOLIN (UNII: 24H4NWX5CO) POLYACRYLIC ACID (800000 MW) (UNII: D0I6NSZ87U) PANAX NOTOGINSENG ROOT (UNII: GQX1C1175U) HUPERZIA SERRATA (UNII: TLA53E5A4T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TURMERIC (UNII: 856YO1Z64F) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68007-301-01 3 in 1 BOX 04/13/2016 1 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/13/2016 Labeler - GUILIN CHINA RESOURCES TIANHE PHARMACEUTICAL CO LTD (528565427) Establishment Name Address ID/FEI Business Operations GUILIN CHINA RESOURCES TIANHE PHARMACEUTICAL CO LTD 528565427 relabel(68007-301) Establishment Name Address ID/FEI Business Operations FOSHAN AQUA GEL BIOTECH CO LTD 529128763 manufacture(68007-301)