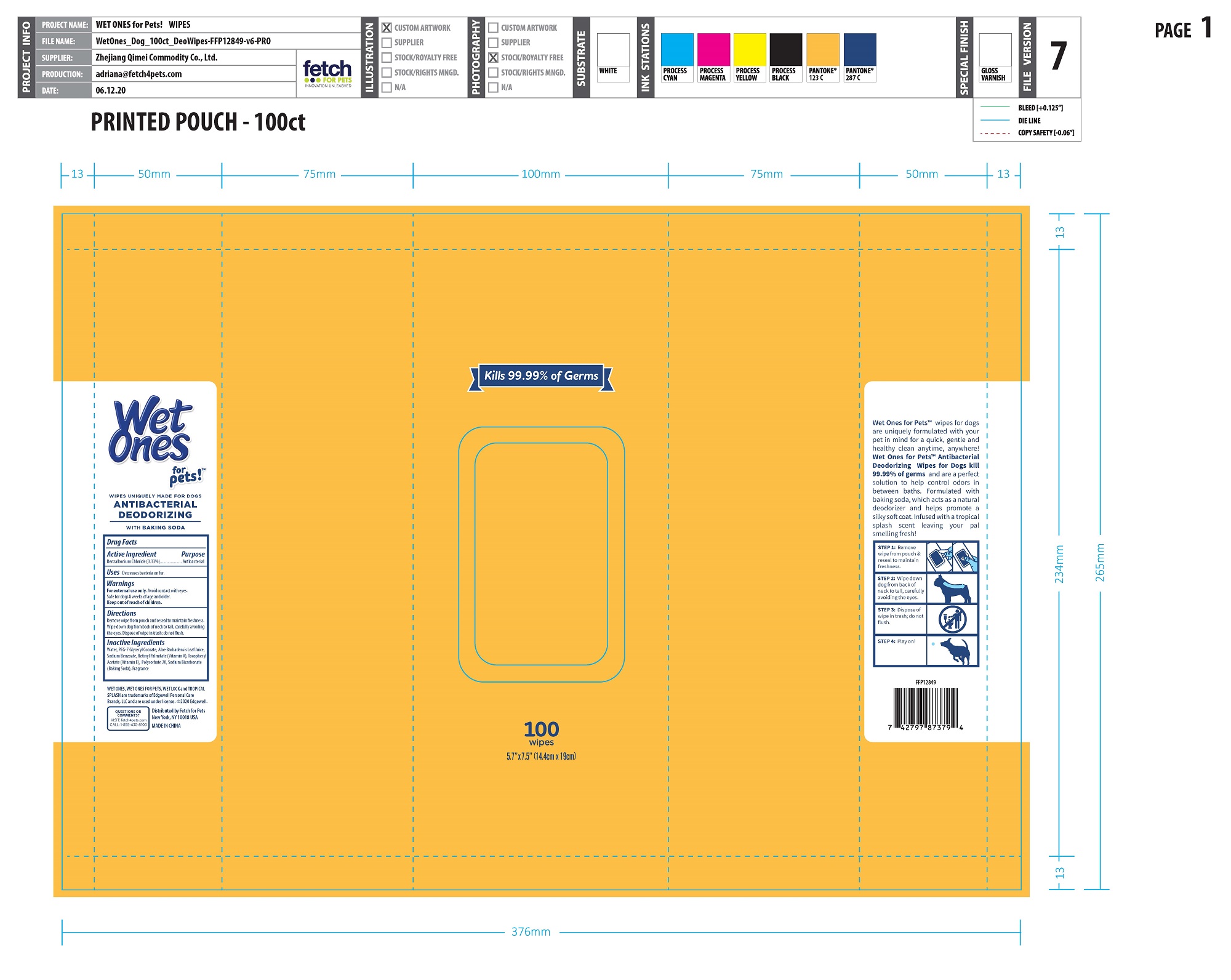
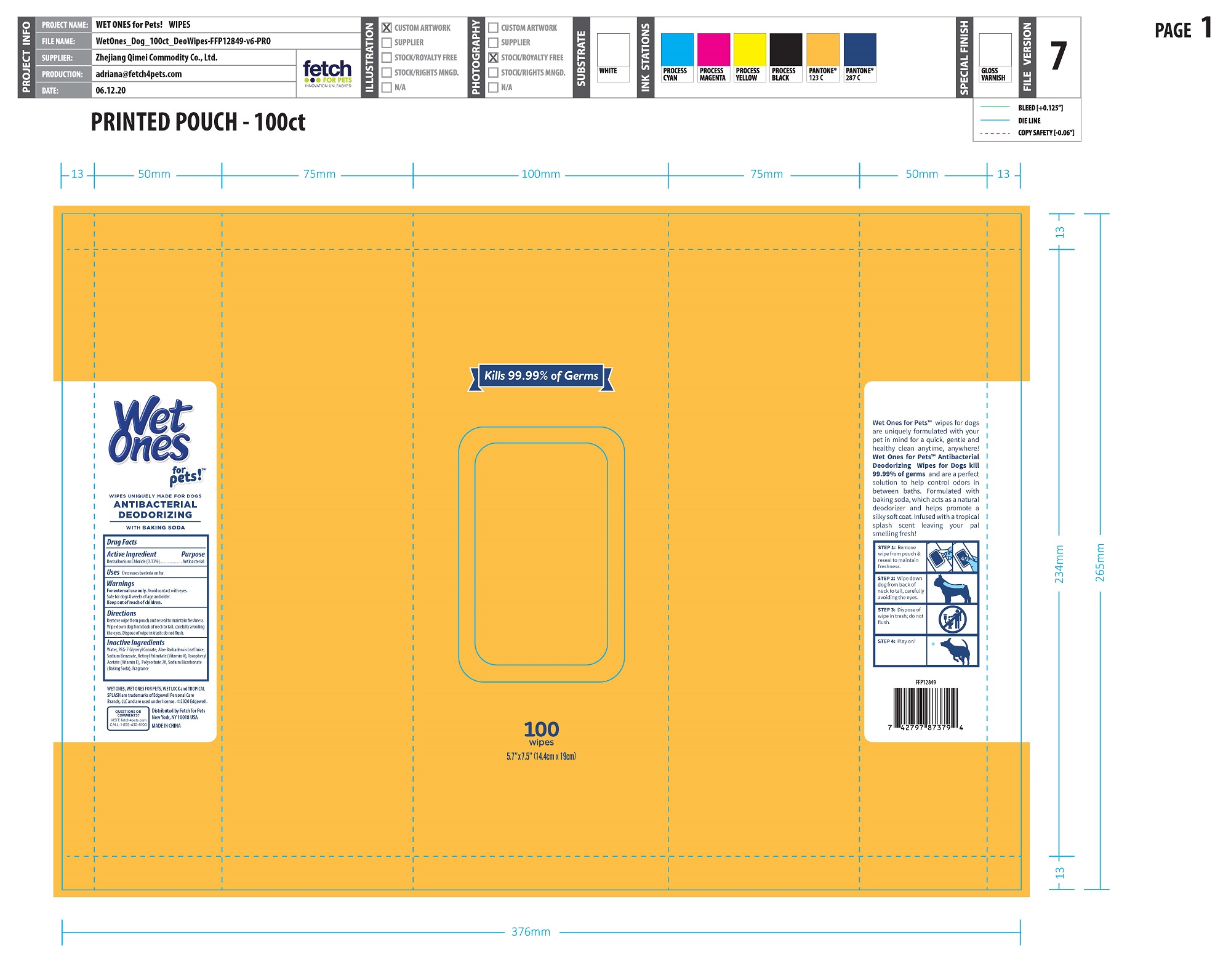
Label: ANTI-BACTERIAL DEODORIZING WIPE FOR DOGS- benzalkonium chloride cloth
-
NDC Code(s):
77720-027-01,
77720-027-02,
77720-027-03,
77720-027-04, view more77720-027-05
- Packager: Skaffles Group Limited Liability Company
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 24, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- USE
- Warning
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI-BACTERIAL DEODORIZING WIPE FOR DOGS
benzalkonium chloride clothProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:77720-027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM BENZOATE (UNII: OJ245FE5EU) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77720-027-01 12 in 1 CARTON 1 50 in 1 CANISTER 1 186 g in 1 NOT APPLICABLE 2 NDC:77720-027-02 24 in 1 CARTON 2 30 in 1 CANISTER 2 112 g in 1 NOT APPLICABLE 3 NDC:77720-027-04 24 in 1 CARTON 3 30 in 1 POUCH 3 112 g in 1 NOT APPLICABLE 4 NDC:77720-027-03 12 in 1 CARTON 4 100 in 1 CANISTER 4 372 g in 1 NOT APPLICABLE 5 NDC:77720-027-05 12 in 1 CARTON 5 100 in 1 POUCH 5 372 g in 1 NOT APPLICABLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/23/2021 Labeler - Skaffles Group Limited Liability Company (831115642) Establishment Name Address ID/FEI Business Operations Zhejiang Qimei Commodity Co.,Ltd. 544331136 manufacture Establishment Name Address ID/FEI Business Operations Lonza Guangzhou Nansha LTD 545328150 api manufacture