Label: ASTHMACARE- safecare liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-4044-2 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 20, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
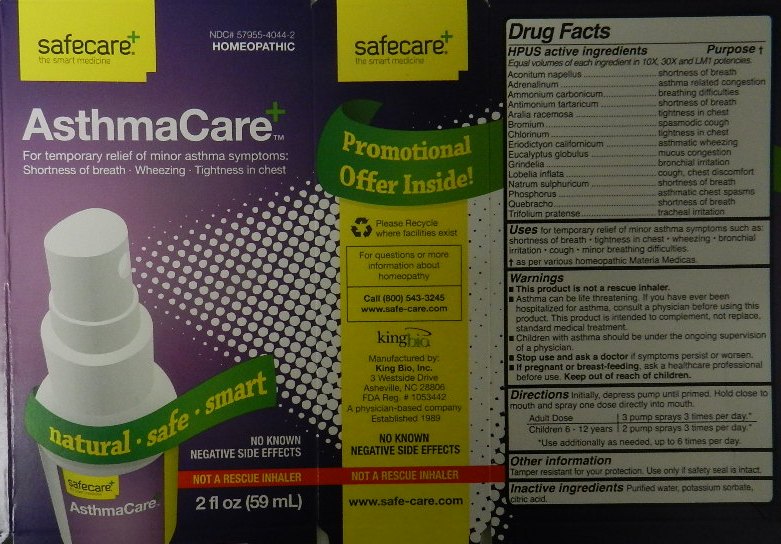
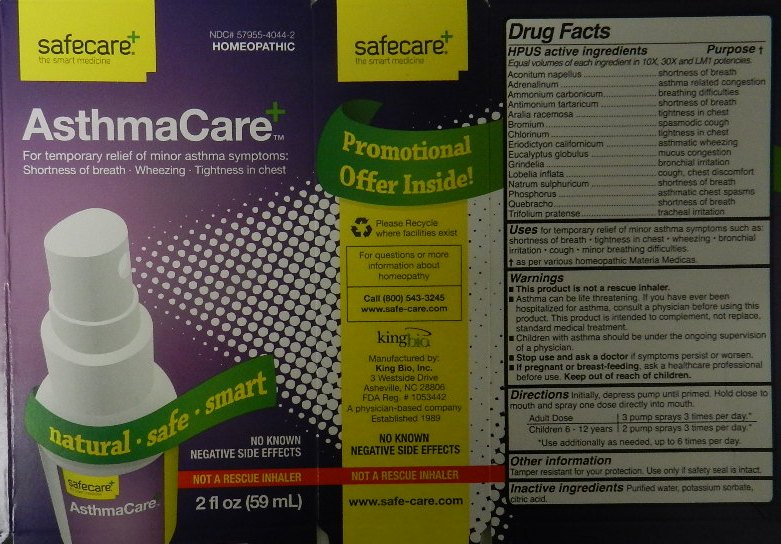
Active Ingredients
HPUS active ingredients
Equal volumes of each ingredient in 10X, 30X and LM1 potencies.
Aconitum napellus
Adrenalinum
Ammonium carbonicum
Antimonium tartaricum
Aralia racemosa
Bromium
Chlorinum
Eriodictyon californicum
Eucalyptus globulus
Grindelia
Lobelia inflata
Natrum sulphuricum
Phosphorus
Quebracho
Trifolium pratense
Reference image asthmacare.jpg
- Inactive Ingredients
-
Purpose
HPUS active ingredients Purpose
Equal volumes of each ingredient in 10X, 30X and LM1 potencies.
Aconitum napellus....................................................................shortness of breath
Adrenalinum.............................................................................asthma related congestion
Ammonium carbonicum............................................................breathing difficulties
Antimonium tartaricum.............................................................shortness of breath
Aralia racemosa.......................................................................tightness in chest
Bromium.................................................................................spasmodic cough
Chlorinum................................................................................tightness in chest
Eriodictyon californicum...........................................................asthmatic wheezing
Eucalyptus globulus.................................................................mucus congestion
Grindelia.................................................................................bronchial irritation
Lobelia inflata..........................................................................cough, chest discomfort
Natrum sulphuricum................................................................shortness of breath
Phosphorus............................................................................asthmatic chest spasms
Quebracho.............................................................................shortness of breath
Trifolium pratense...................................................................tracheal irritation
Reference image asthmacare.jpg
- Dosage and Administration
-
Warnings
- This product is not a rescue inhaler.
- Asthma can be life threatening. If you have ever been hospitalized for asthma, consult a physician before using this product. This product is intended to complement, not replace, standard medical treatment.
- Children with asthma should be under the ongoing supervision of a physician.
- Stop use and ask a doctor if symptoms persist or worsen.
- If pregnant or breast-feeding, ask a healthcare professional before use. Keep out of reach of children.
Tamper resistant for your protection. Use only if safety seal is intact.
Reference image asthmacare.jpg
- KEEP OUT OF REACH OF CHILDREN
- Indications and Usage
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ASTHMACARE
safecare liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-4044 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 10 [hp_X] in 59 mL EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 10 [hp_X] in 59 mL AMMONIUM CARBONATE (UNII: NJ5VT0FKLJ) (AMMONIUM CATION - UNII:54S68520I4) AMMONIUM CARBONATE 10 [hp_X] in 59 mL ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 10 [hp_X] in 59 mL ARALIA RACEMOSA ROOT (UNII: T90W4582DU) (ARALIA RACEMOSA ROOT - UNII:T90W4582DU) ARALIA RACEMOSA ROOT 10 [hp_X] in 59 mL BROMINE (UNII: SBV4XY874G) (BROMINE - UNII:SBV4XY874G) BROMINE 10 mL in 59 mL CHLORINE (UNII: 4R7X1O2820) (CHLORINE - UNII:4R7X1O2820) CHLORINE 10 [hp_X] in 59 mL ERIODICTYON CALIFORNICUM LEAF (UNII: 2Y7TIQ135H) (ERIODICTYON CALIFORNICUM LEAF - UNII:2Y7TIQ135H) ERIODICTYON CALIFORNICUM LEAF 10 [hp_X] in 59 mL EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 10 [hp_X] in 59 mL GRINDELIA HIRSUTULA FLOWERING TOP (UNII: IDB0NAZ6AI) (GRINDELIA HIRSUTULA FLOWERING TOP - UNII:IDB0NAZ6AI) GRINDELIA HIRSUTULA FLOWERING TOP 10 [hp_X] in 59 mL LOBELIA INFLATA (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA 10 [hp_X] in 59 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM SULFATE 10 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] in 59 mL QUEBRACHO BARK (UNII: HST8772GTZ) (QUEBRACHO BARK - UNII:HST8772GTZ) QUEBRACHO BARK 10 [hp_X] in 59 mL TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) (TRIFOLIUM PRATENSE FLOWER - UNII:4JS0838828) TRIFOLIUM PRATENSE FLOWER 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-4044-2 1 in 1 CARTON 1 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/14/2012 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 manufacture