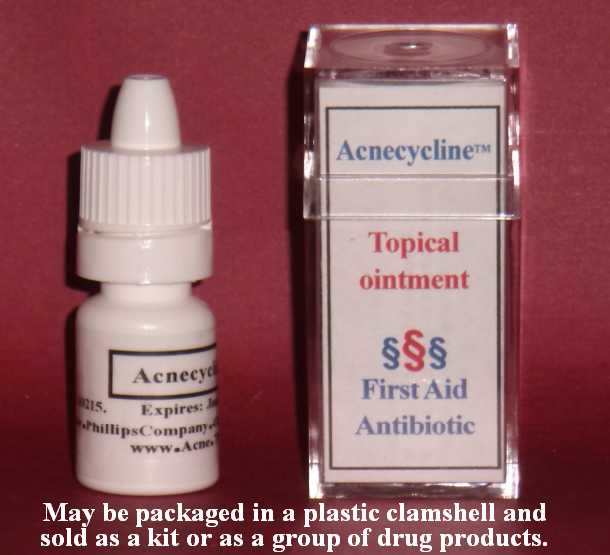
Label: ACNECYCLINE- tetracycline hydrochloride ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 43074-116-01, 43074-116-02 - Packager: Phillips Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 15, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DRUG FACTS
DRUG FACTS
Active Ingredient Purpose
Tetracycline (3%) ....................... First-aid antibiotic
Uses
First aid to help prevent skin infection in minor cuts, scrapes and burns
For external use only.
Keep away from children.
Do not use in the eyes or apply over large areas of the body.
May be harmful if swallowed.
In case of deep or puncture wounds, animal bites, or serious burns, consult a physician.
Stop use and consult a physician if the condition persists or gets worse.
Do not use if allergic to any ingredient listed on this label.
Directions
Clean the affected area. Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area and rub gently using a cotton swab. If not completely rubbed in after a 20-second rub-in, too much product was used. Repeat the process three times daily. Keep this product refrigerated to preserve effectiveness and color.
Other information
This product is an OTC antibiotic drug product for human use. Blended for typical skin color. May stain cloth.
Inactive ingredients
Water, glycerin, hydroxethylcellulose, chlor-hexidine gluconate, glucono delta lactone, methylparaben, sodium hydroxide, dipropylene glycol, dimethyl sulfoxide, sorbic acid, ascorbic acid, magnesium stearate, stearic acid.
Side effects
Same as other tetracycline products.
See listing of side effects online at
www.PhillipsCompany.4T.com/CC.pdf
Report any side effects to Phillips Company,
311 Chickasaw Street, Millerton, OK USA 74750
Tel. 580-746-2430.
Email: PhillipsCompany@cox.net
- ACTIVE INGREDIENTS
- ASK DOCTOR
- DO NOT USE
- CHILDREN
- PURPOSE
- STOP USE
- WHEN USING
- WARNINGS
-
DOSAGE & ADMINISTRATION
Clean the affected area. Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area and rub gently using a cotton swab. If not completely rubbed in after a 20-second rub-in, too much product was used. Repeat the process three times daily. For best results, refrigerate this product to reduce oxidation rate; to preserve effectiveness and color (normally orange). Discard this product if color turns black.
-
USE
First aid to help prevent skin infection in minor cuts, scrapes and burns
For external use only.
Keep away from children.
Do not use in the eyes or apply over large areas of the body.
May be harmful if swallowed.
In case of deep or puncture wounds, animal bites, or serious burns, consult a physician.
Stop use and consult a physician if the condition persists or gets worse.
Do not use if allergic to any ingredient listed on this label.
- INACTIVE INGREDIENTS
- Image of product
-
INGREDIENTS AND APPEARANCE
ACNECYCLINE
tetracycline hydrochloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43074-116 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACYCLINE HYDROCHLORIDE (UNII: P6R62377KV) (TETRACYCLINE - UNII:F8VB5M810T) TETRACYCLINE 0.03 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ascorbic acid (UNII: PQ6CK8PD0R) dipropylene glycol (UNII: E107L85C40) Water (UNII: 059QF0KO0R) glycerin (UNII: PDC6A3C0OX) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) calcium gluconate (UNII: SQE6VB453K) methylparaben (UNII: A2I8C7HI9T) sodium hydroxide (UNII: 55X04QC32I) sorbic acid (UNII: X045WJ989B) magnesium stearate (UNII: 70097M6I30) stearic acid (UNII: 4ELV7Z65AP) SODIUM BICARBONATE (UNII: 8MDF5V39QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43074-116-02 1 in 1 BOTTLE, PLASTIC 1 NDC:43074-116-01 3 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 02/15/2011 Labeler - Phillips Company (612368238) Establishment Name Address ID/FEI Business Operations Phillips Company 612368238 manufacture