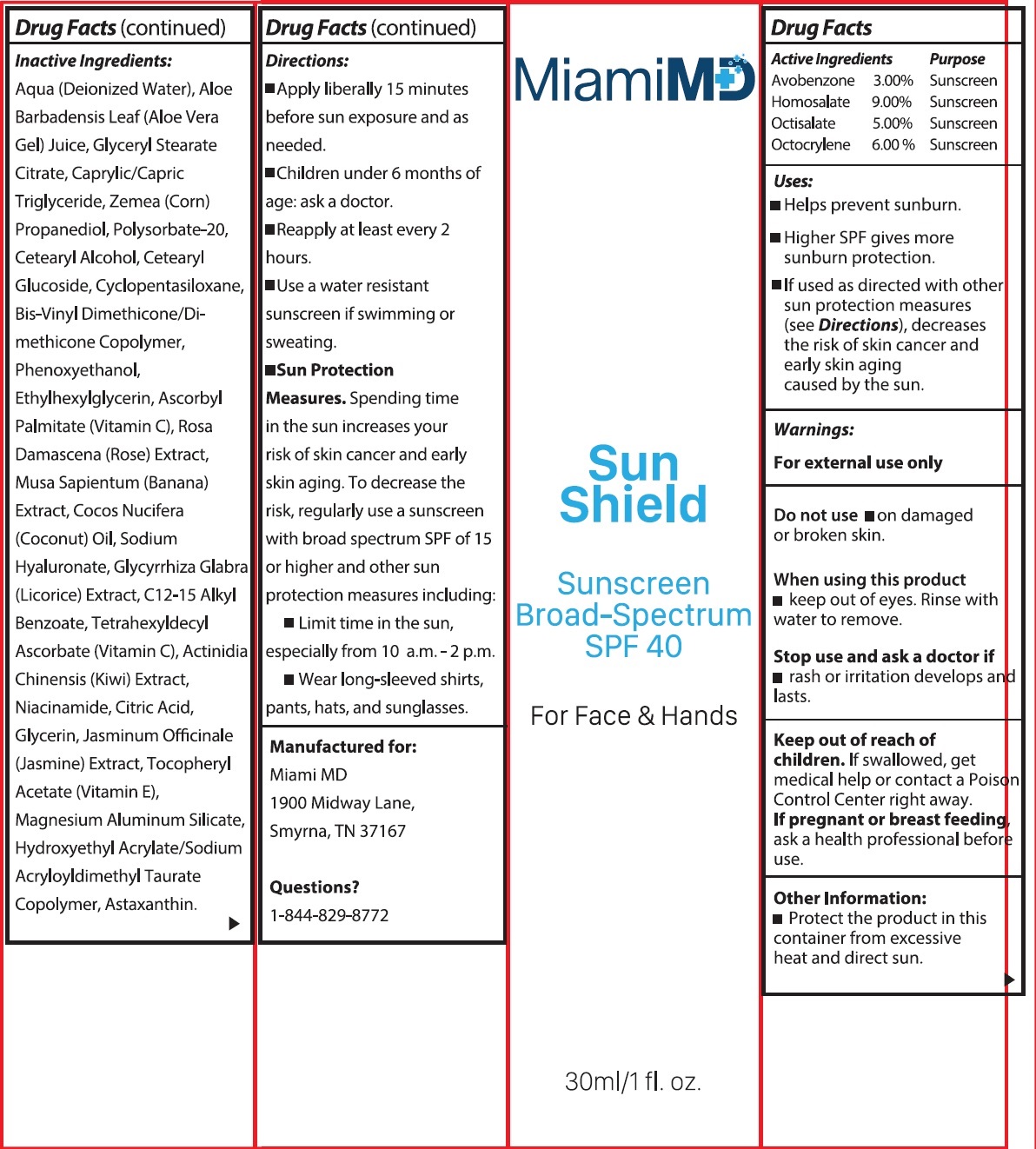
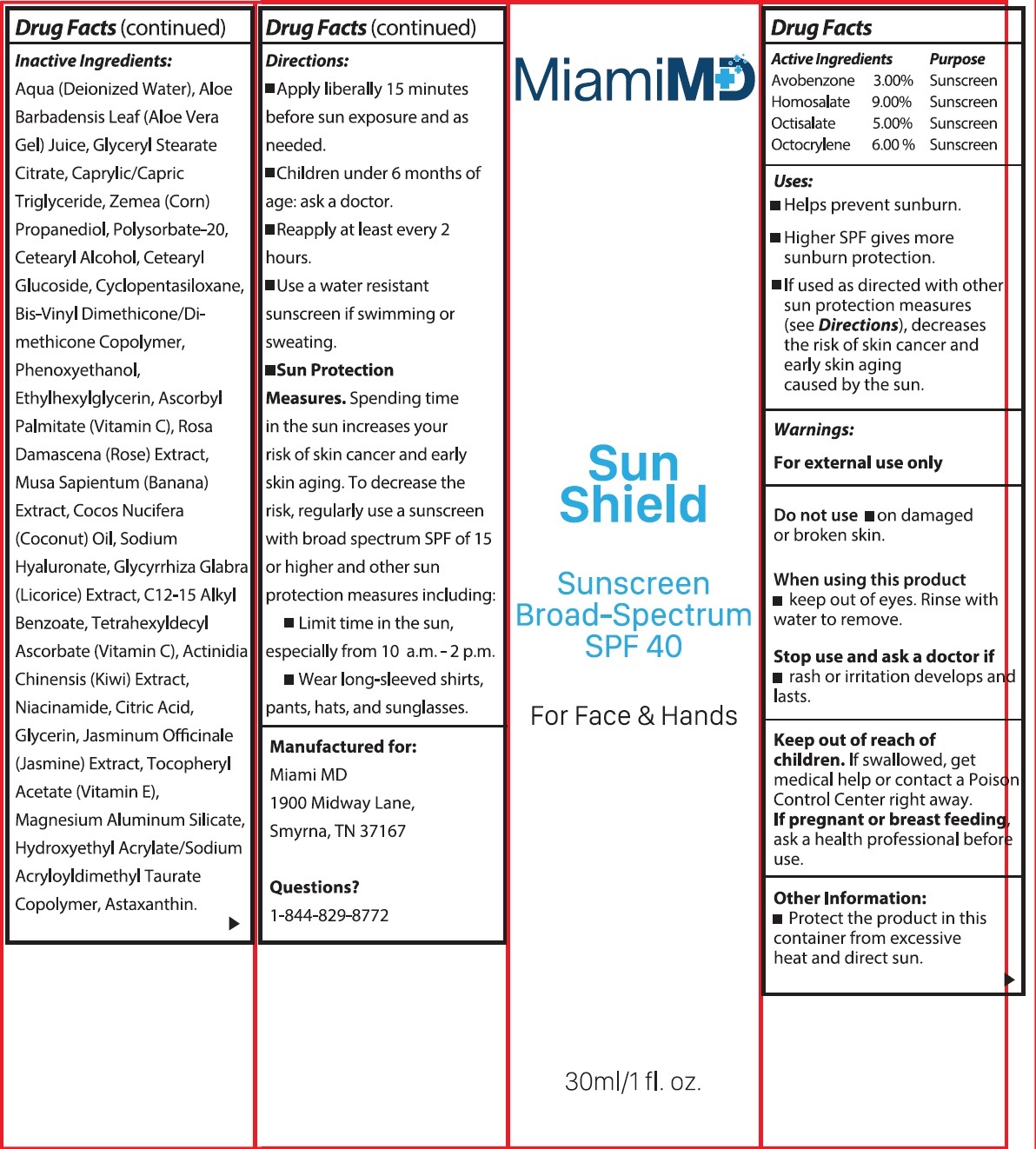
Label: MIAMI MD SUN SHIELD BROAD SPECTRUM SPF 40- avobenzone, homosalate, octisalate, octocrylene cream
- NDC Code(s): 83140-373-00
- Packager: BRAND BOLT LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses:
- Warnings
- Other Information:
-
Inactive Ingredients:
Aqua (Deionized Water), Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Glyceryl Stearate Citrate, Caprylic/Capric Triglyceride, Zemea (Corn) Propanediol, Polysorbate-20, Cetearyl Alcohol, Cetearyl Glucoside, Cyclopentasiloxane, Bis-Vinyl Dimethicone/Dimethicone Copolymer, Phenoxyethanol, Ethylhexylglycerin, Ascorbyl Palmitate (Vitamin C), Rosa Damascena (Rose) Extract, Musa Sapientum (Banana) Extract, Cocos Nucifera (Coconut) Oil, Sodium Hyaluronate, Glycyrrhiza Glabra (Licorice) Extract, C12-15 Alkyl Benzoate, Tetrahexyldecyl Ascorbate (Vitamin C), Actinidia Chinensis (Kiwi) Extract, Niacinamide, Citric Acid, Glycerin, Jasminum Officinale (Jasmine) Extract, Tocopheryl Acetate (Vitamin E), Magnesium Aluminum Silicate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Astaxanthin.
-
Directions
- Apply liberally 15 minutes before sun exposure and as needed.
- Children under 6 months of age: ask a doctor.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures.
- Limit time in the sunm especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MIAMI MD SUN SHIELD BROAD SPECTRUM SPF 40
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83140-373 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 90 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CORN (UNII: 0N8672707O) POLYSORBATE 20 (UNII: 7T1F30V5YH) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ASCORBYL PALMITATE (UNII: QN83US2B0N) BANANA (UNII: 4AJZ4765R9) COCONUT OIL (UNII: Q9L0O73W7L) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KIWI FRUIT (UNII: 71ES77LGJC) NIACINAMIDE (UNII: 25X51I8RD4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) ASTAXANTHIN (UNII: 8XPW32PR7I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83140-373-00 1 in 1 CARTON 12/05/2022 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/05/2022 Labeler - BRAND BOLT LLC (118171618)