Label: METOPIRONE- metyrapone capsule, gelatin coated
- NDC Code(s): 76336-455-18
- Packager: HRA Pharma Rare Diseases
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use METOPIRONE safely and effectively. See full prescribing information for METOPIRONE.
METOPIRONE® (metyrapone capsules), for oral use
Initial U.S. Approval: 1961RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Metopirone is indicated, in combination with other diagnostic tests, for the diagnosis of adrenal insufficiency in adult and pediatric patients. (1)
DOSAGE FORMS AND STRENGTHS
Capsules: 250 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Adrenal Insufficiency: May induce acute adrenal insufficiency. Ability of adrenals to respond to exogenous ACTH should be demonstrated before Metopirone is employed as a test (5.1)
- Dizziness and Sedation: May cause dizziness and sedation. Patients should not drive or operate machinery until these effects have passed (5.2)
ADVERSE REACTIONS
Adverse reactions include: hypotension, nausea, vomiting, abdominal discomfort or pain, headache, dizziness, sedation, allergic rash, leukopenia, anemia, and/or thrombocytopenia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Direct Success Inc. at 844-597-6373 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Information Before Conducting Metopirone Testing
2.2 Single-Dose Short Test- Recommended Dose and Interpretation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Adrenal Insufficiency
5.2 Dizziness and Sedation
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Metopirone
7.2 Effect of Metopirone on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Information Before Conducting Metopirone Testing
Stop drugs affecting pituitary or adrenocortical function before administration of Metopirone in accordance with half-life of the drugs (consider at least 5 half-lives to avoid any interference with Metopirone testing). [see Drug Interactions (7.1)].
Assess ability of patient's adrenals to respond to exogenous ACTH before Metopirone is employed as a test [see Warnings and Precautions (5.1)].
2.2 Single-Dose Short Test- Recommended Dose and Interpretation
This test, usually given on an outpatient basis, determines plasma 11-desoxycortisol and/or ACTH levels after a single dose of Metopirone. Patients with suspected adrenocortical insufficiency based on the test results previously performed should be hospitalized overnight as a precautionary measure (see Warnings and Precaution (5.1)).
Recommended Dose
In adult and pediatric patients, the recommended single dose is 30 mg/kg (maximum 3 grams of Metopirone) administered at midnight with milk/yogurt or snack. The blood sample for the assay is taken early the following morning (7:30-8:00 a.m.). After the blood sample is collected, a prophylactic dose of glucocorticoid may be considered for patients with high risk for acute adrenal insufficiency.
Interpretation of 11‑desoxycortisol and ACTH Levels After Metopirone Administration
Approximately 8 hours after administration of Metopirone, evaluate the values of ACTH and 11-desoxycortisol. Normal values will depend on the method used to determine ACTH and 11‑desoxycortisol levels. An intact HPA axis function is generally indicated by an increase in 11‑desoxycortisol to over 70 mcg/L. Because of an overlap between a normal ACTH response and an abnormal ACTH response, the ACTH response alone cannot be used to distinguish between healthy individuals and those with adrenal insufficiency.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Adrenal Insufficiency
Metopirone may induce acute adrenal insufficiency in patients with reduced adrenal secretory capacity, as well as in patients with global pituitary insufficiency. The test should be performed in the hospital with close monitoring in case of suspected adrenal insufficiency.
Ability of adrenals to respond to exogenous ACTH should be demonstrated before Metopirone is employed as a test.
In the presence of hypo‑ or hyperthyroidism, response to the Metopirone test may be subnormal.
If adrenocortical or anterior pituitary function is more severely compromised than indicated by the results of the test, Metopirone may trigger adrenal insufficiency. This can be corrected by giving appropriate doses of corticosteroids.
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of Metopirone were identified in clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular System: Hypotension
- Gastrointestinal System: Nausea, vomiting, abdominal discomfort or pain
- Central Nervous System: Headache, dizziness, sedation
- Dermatologic System: Allergic rash
- Hematologic System: Leukopenia, anemia, and/or thrombocytopenia
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Metopirone
Anticonvulsants, psychotropic drugs, hormone preparations, corticosteroids, antithyroid agents and cyproheptadine may affect the results of the Metopirone test.
If these drugs cannot be withdrawn, the necessity of carrying out the Metopirone test should be reviewed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published case series and reports on Metopirone use in pregnant females are insufficient to identify a drug-associated risk of major birth defects or miscarriage. Metyrapone crosses the placenta and may decrease fetal cortisol production (see Data). Animal reproductive studies have not been conducted with metyrapone. Metyrapone can decrease reproductive hormones by targeting adrenal androgenesis.
Data
Human Data
The Metopirone test was administered to pregnant women in their second and third trimester of pregnancy and evidence was found that the fetal pituitary responded to the enzymatic block. Transplacental transfer of Metopirone has been shown in humans and the drug can impair the biosynthesis of fetal and placental steroids. There are a few published reports of low cortisol levels at birth in infants exposed in utero following chronic use of metyrapone in pregnant females.
8.2 Lactation
Risk Summary
Metyrapone and its active metabolite, metyrapol, are present in human milk. There are no available data on the effects of metyrapone on the breastfed infant or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Metopirone and any potential adverse effects on the breastfed infant from Metopirone or from the underlying maternal condition.
8.4 Pediatric Use
Metopirone is indicated, in combination with other diagnostic tests, for the diagnosis of adrenal insufficiency in pediatric patients [See Dosage and Administration (2.1)].
8.5 Geriatric Use
Clinical studies of Metopirone did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
Other reported clinical experience has not identified differences in responses between patients 65 years of age and older and younger adult patients.
-
10 OVERDOSAGE
Death occurred in a child after two doses of Metopirone 2 g.
Signs and Symptoms of Overdosage
The clinical picture of overdosage with Metopirone is characterized by gastrointestinal symptoms and by signs of acute adrenal insufficiency.
- -
- Cardiovascular System: Cardiac arrhythmias, hypotension, dehydration.
- -
- Nervous System and Muscles: Anxiety, confusion, weakness, impairment of consciousness.
- -
- Gastrointestinal System: Nausea, vomiting, epigastric pain, diarrhea.
- -
- Laboratory Findings: Hyponatremia, hypochloremia, hyperkalemia.
In patients under treatment with insulin or oral antidiabetics, the signs and symptoms of acute overdosage with Metopirone may be aggravated or modified.
Treatment and Management of Overdosage
There is no specific antidote for Metopirone overdosage. Immediate treatment is essential in the management of Metopirone overdose.
Treatment with activated charcoal may be considered if the overdose has been taken within 1 hour. In addition to general measures, hydrocortisone should be administered at once, together with IV saline and glucose. This should be repeated as necessary in accordance with the patient's clinical condition.
For a few days blood pressure and fluid and electrolyte balance should be monitored.
Consult with a regional poison control center (1-800-222-1222) for additional treatment recommendations.
-
11 DESCRIPTION
Metopirone (metyrapone capsules) is an adrenal steroid synthesis inhibitor, available as 250‑mg capsules for oral administration. Its chemical name is 2‑methyl‑1, 2‑di‑3‑pyridyl‑1‑propanone, and its structural formula is
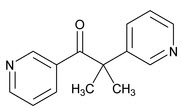
Metyrapone is a white to light amber, fine, crystalline powder, having a characteristic odor. It is sparingly soluble in water, and soluble in methanol and in chloroform. It forms water‑soluble salts with acids. Its molecular weight is 226.27.
Inactive Ingredients: Ethyl vanillin, gelatin, glycerol, macrogol 400, macrogol 4000, paramethoxy acetophenone, purified water, sodium ethyl parahydroxybenzoate, sodium propyl parahydroxybenzoate, titanium dioxide, + red ink (aluminum chloride hexahydrate, carminic acid, hypromellose, propylene glycol, sodium hydroxide).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The pharmacological effect of Metopirone is to reduce cortisol and corticosterone production by inhibiting the 11-beta-hydroxylation reaction in the adrenal cortex. Removal of the strong inhibitory feedback mechanism exerted by cortisol results in an increase in adrenocorticotropic hormone (ACTH) production by the pituitary. With continued blockade of the enzymatic steps leading to production of cortisol and corticosterone, there is a marked increase in adrenocortical secretion of their immediate precursors, 11-desoxycortisol and desoxycorticosterone, which are weak suppressors of ACTH release, and a corresponding elevation of these steroids in the plasma and of their metabolites in the urine. These metabolites are readily determined by measuring urinary 17‑hydroxycorticosteroids (17-OHCS) or 17-ketogenic steroids (17-KGS). Because of these actions, Metopirone is used as a diagnostic test, with urinary 17-OHCS measured as an index of pituitary ACTH responsiveness. Metopirone may also suppress biosynthesis of aldosterone, resulting in a mild natriuresis.
12.2 Pharmacodynamics
Metopirone exposure-response relationships and the time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The mean Cmax of metyrapone following a single 750 mg is 3.7 mcg/mL falling to 0.5 mcg/mL 4 hours after administration.
Absorption
The time to peak plasma concentration of metyrapone is approximately 1 hour after oral administration.
Elimination
The mean (± SD) terminal elimination half‑lives of metyrapone and its active metabolite metyrapol are 1.9 (± 0.7) hours and approximately 4 hours, respectively.
- 13 NONCLINICAL TOXICOLOGY
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise patient to read the FDA-approved labeling (Patient Information).
Advise patient that Metopirone may induce acute adrenal insufficiency (nausea, vomiting, abdominal pain, hypotension) [see Warnings and Precautions (5.1)].
Advise patient that Metopirone may cause dizziness and sedation. Advise patients not to drive or operate machinery until these effects have passed [see Warnings and Precautions (5.2)].
-
SPL UNCLASSIFIED SECTION
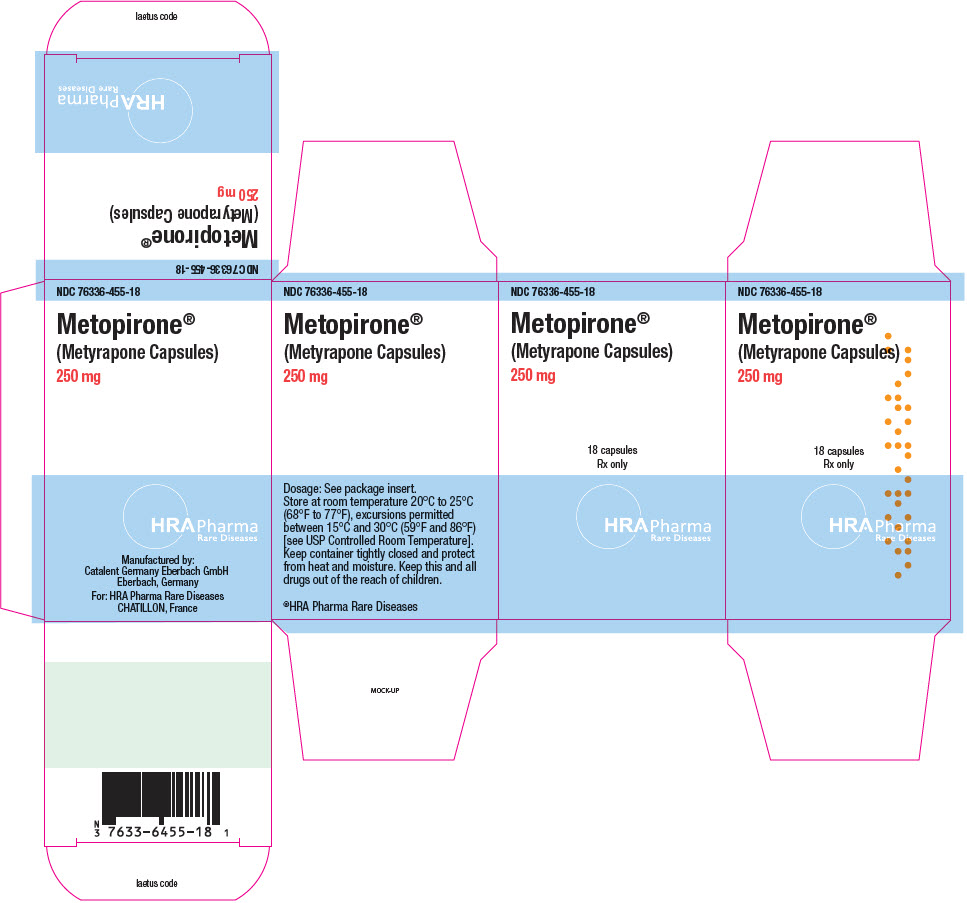
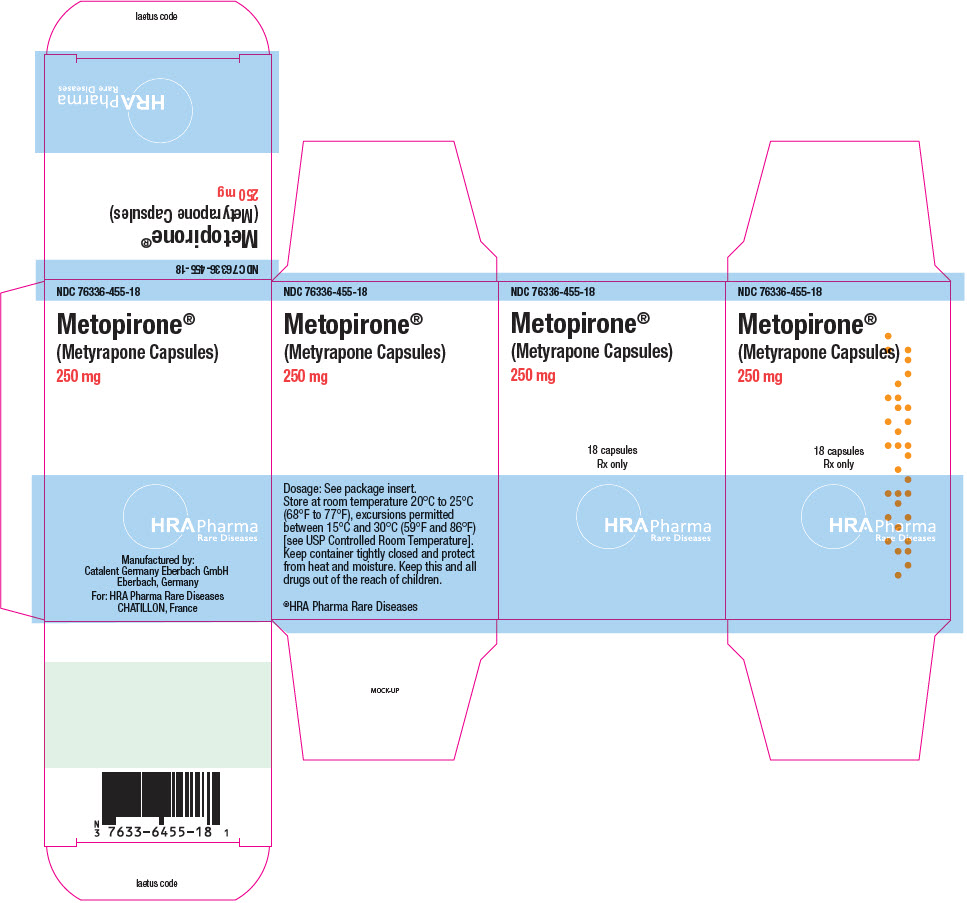
Manufactured by:
Catalent Germany Eberbach GmbH
Eberbach, GermanyFor:
HRA Pharma Rare Diseases
CHATILLON, France© 2022 HRA Pharma Rare Diseases | Metopirone is a registered trademark of HRA Pharma Rare Diseases
Address medical inquiries to:
Direct Success Inc.
1710 Hwy 34
Farmingdale, NJ 07727
1-844-597-6373
Fax: 1-855-674-6767 - PRINCIPAL DISPLAY PANEL - 250 mg Bottle Carton
-
INGREDIENTS AND APPEARANCE
METOPIRONE
metyrapone capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76336-455 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METYRAPONE (UNII: ZS9KD92H6V) (METYRAPONE - UNII:ZS9KD92H6V) METYRAPONE 250 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) GLYCERIN (UNII: PDC6A3C0OX) ETHYLPARABEN SODIUM (UNII: Z0D00IVA10) 4-ACETYLANISOLE (UNII: 0IRH2BR587) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) PROPYLPARABEN SODIUM (UNII: 625NNB0G9N) ETHYL VANILLIN (UNII: YC9ST449YJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (white to yellowish-white) Score no score Shape CAPSULE (soft gelatin; oblong; opaque) Size 19mm Flavor Imprint Code HRA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76336-455-18 18 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/1962 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA012911 01/25/1962 Labeler - HRA Pharma Rare Diseases (571682231) Establishment Name Address ID/FEI Business Operations Minakem Beuvry Production 265413258 ANALYSIS(76336-455) , API MANUFACTURE(76336-455) Establishment Name Address ID/FEI Business Operations Minakem Dunkerque Production 277412599 ANALYSIS(76336-455) Establishment Name Address ID/FEI Business Operations Catalent Germany Eberbach GmbH 318612223 MANUFACTURE(76336-455) , ANALYSIS(76336-455) Establishment Name Address ID/FEI Business Operations DELPHARM LILLE SAS 283582547 PACK(76336-455) , ANALYSIS(76336-455) Establishment Name Address ID/FEI Business Operations Piramal Pharma Limited 919067108 ANALYSIS(76336-455) , API MANUFACTURE(76336-455)