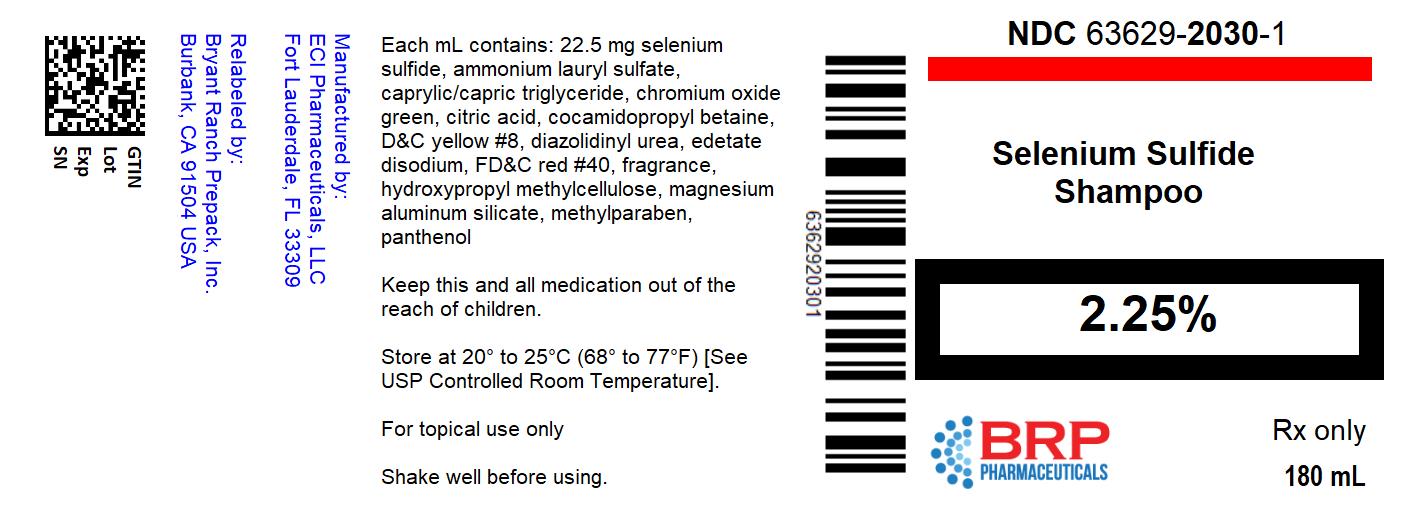
Label: SELENIUM SULFIDE shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 63629-2030-1 - Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 70156-111
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DIRECTIONS
A liquid antiseborrehic, antifungal preparation for topical application.
Each mL of Selenium Sulfide 2.25% Shampoo contains 22.5 mg selenium sulfide, ammonium lauryl sulfate, caprylic/capric triglyceride, chromium oxide green, citric acid, cocamidopropyl betaine, D&C yellow #8, diazolidinyl urea, edetate disodium, FD&C red #40, fragrance, hydroxypropyl methylcellulose, magnesium aluminum silicate, methylparaben, panthenol, PPG-2 hydroxyethyl coco/isostearamide, propylene glycol, propylparaben, purified water, sodium citrate, titanium dioxide, tocopheryl acetate, urea, zinc pyrithione.
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
This medication is to be used as directed by a physician. Not to be used when inflammation or exudation is present as increased absorption may occur.
CARCINOGENESIS
Dermal application of 25% and 50% solutions of 2.5% selenium sulfide lotion on mice over an 88-week period indicated no carcinogenic effects.
USE IN PREGNANCY
CATEGORY C
Animal reproduction studies have not been conducted with this medication. It is also not known whether this product can cause fetal harm when applied to the body surfaces of a pregnant woman or can affect reproduction capacity. Under ordinary circumstances, selenium sulfide 2.25% shampoo should not be used by pregnant women.
- ADVERSE REACTIONS
-
OVERDOSAGE
There are no documented reports of serious toxicity in humans resulting from acute ingestion of selenium sulfide 2.25% shampoo. However, acute toxicity studies in animals suggest that ingestion of large amounts could result in potential human toxicity. Evacuation of the stomach contents should be considered in cases of acute oral ingestion.
-
DOSAGE AND ADMINISTRATION
SHAKE WELL BEFORE USING
For seborrheic dermatitis and dandruff
Generally 2 applications each week for 2 weeks will control symptoms. Subsequently, shampoo may be used less frequently – weekly, every 2 weeks, every 3 to 4 weeks or as directed by a physician. Should not be applied more frequently than necessary to maintain control.
-
HOW SUPPLIED
Selenium Sulfide 2.25% Shampoo is supplied in 180 mL bottles, NDC 63629-2030-1.
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized. Protect from freezing.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SELENIUM SULFIDE
selenium sulfide shampooProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-2030(NDC:70156-111) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM SULFIDE - UNII:Z69D9E381Q) SELENIUM SULFIDE 22.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) TRICAPRIN (UNII: O1PB8EU98M) CHROMIC OXIDE (UNII: X5Z09SU859) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) FLUORESCEIN SODIUM (UNII: 93X55PE38X) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYLPARABEN (UNII: A2I8C7HI9T) PANTHENOL (UNII: WV9CM0O67Z) PPG-2 HYDROXYETHYL COCO/ISOSTEARAMIDE (UNII: EK4J71ZKEQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) UREA (UNII: 8W8T17847W) PYRITHIONE ZINC (UNII: R953O2RHZ5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-2030-1 1 in 1 CARTON 05/09/2017 1 180 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 05/09/2017 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-2030) , RELABEL(63629-2030)