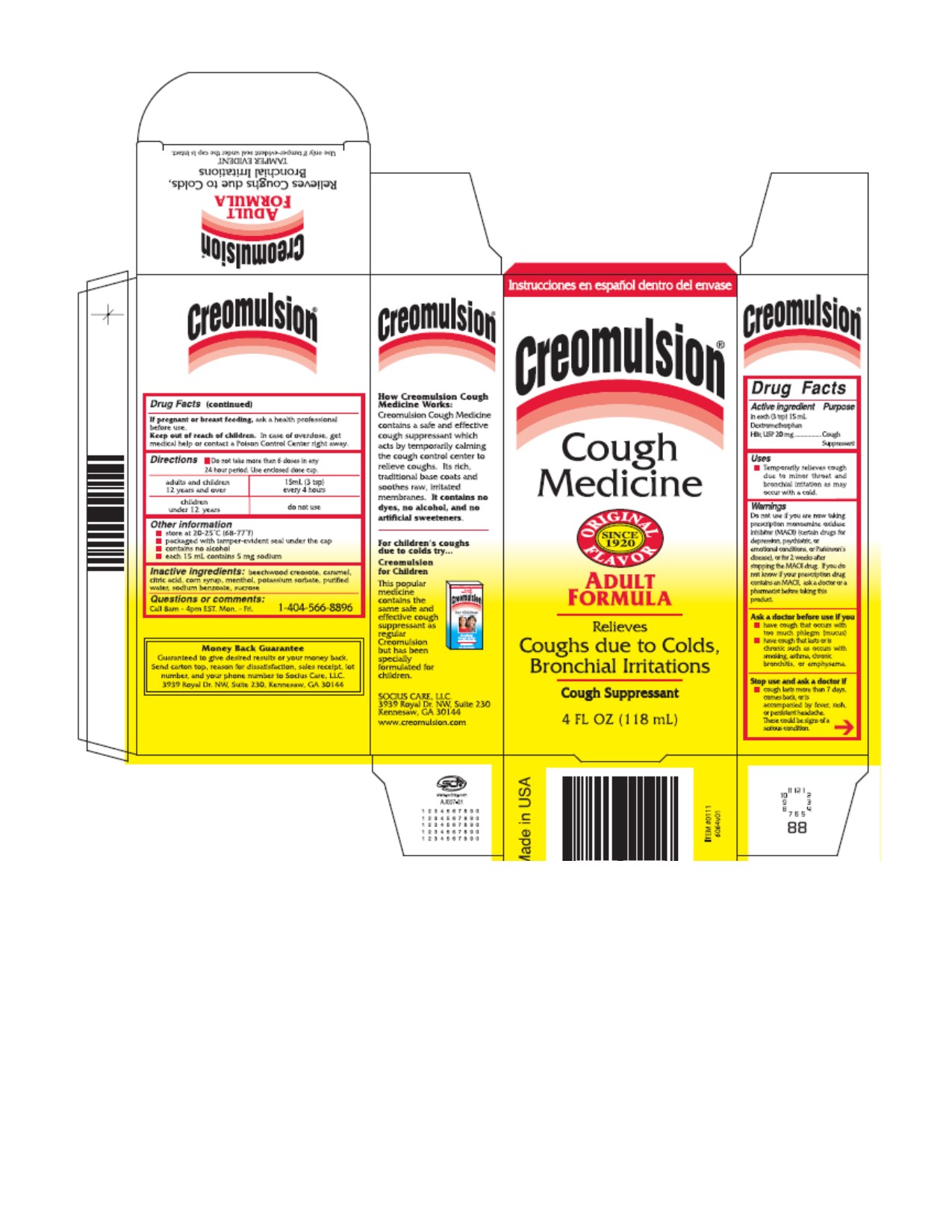
Label: CREOMULSION COUGH MEDICINE ADULT FORMULA- dextromethorphan hydrobromide syrup
- NDC Code(s): 70893-0011-0, 70893-0011-1
- Packager: Socius Care, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
Do not use if you are now taking prescription monoamine oxidase inhibitor (MAOI)
(certain drugs for depression, psychiatric; or emotional conditions, or Parkinson's disease),
or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or a pharmacist before taking this product.
- Ask a doctor before use if you
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive ingredients
- Questions or Comments
-
DESCRIPTION
How Creomulsion Cough Medicine Works?
The Creomulsion Cough Medicine contains a safe and effective cough suppressant which acts by temporarily calming the cough control center to relieve coughs. Its rich, traditional base coats and soothes raw, irritated membranes. It contains no dyes, no alcohol, and no artificial sweeteners.
- Image of Carton and Label
-
INGREDIENTS AND APPEARANCE
CREOMULSION COUGH MEDICINE ADULT FORMULA
dextromethorphan hydrobromide syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70893-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 15 mL Inactive Ingredients Ingredient Name Strength WOOD CREOSOTE (UNII: 3JYG22FD73) CARAMEL (UNII: T9D99G2B1R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CORN SYRUP (UNII: 9G5L16BK6N) MENTHOL (UNII: L7T10EIP3A) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70893-0011-0 1 in 1 CARTON 08/11/2016 1 NDC:70893-0011-1 118 mL in 1 BOTTLE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/11/2016 Labeler - Socius Care, LLC (079946712)