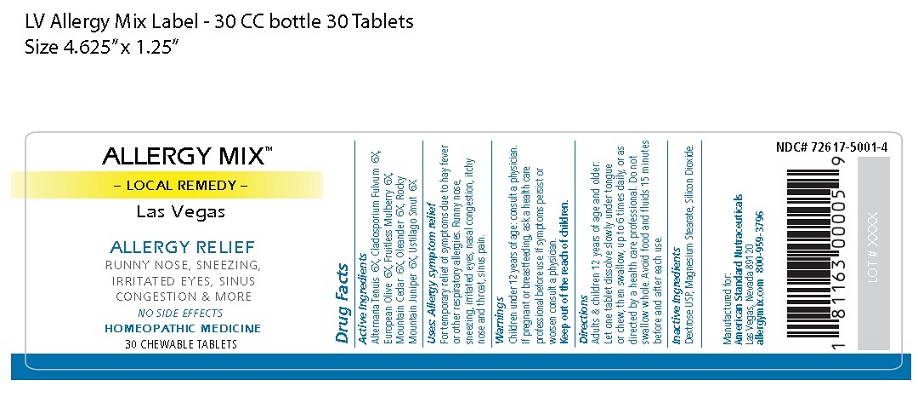
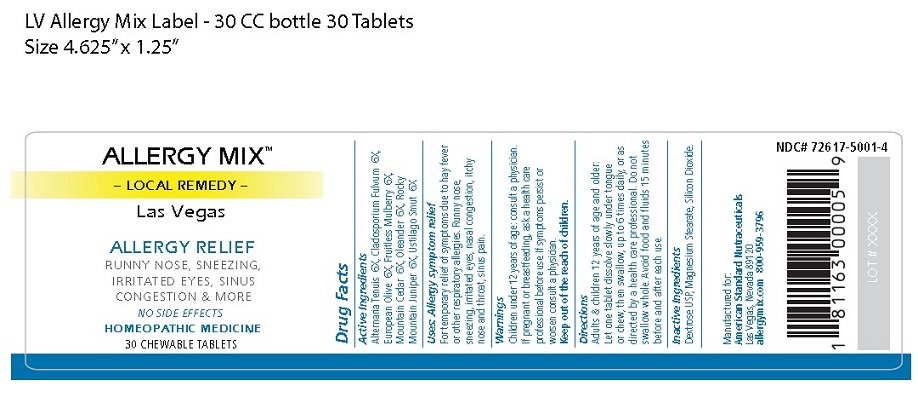
Label: ALLERGY MIX LAS VEGAS- local remedy las vegas tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 72617-5001-4 - Packager: ASN
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 11, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
Principal Display Package
ALLERGY MIX™
– LOCAL REMEDY –
Las VegasALLERGY RELIEF
RUNNY NOSE, SNEEZING,
IRRITATED EYES, SINUS
CONGESTION & MORENO SIDE EFFECTS
HOMEOPATHIC MEDICINE
30 CHEWABLE TABLETS
NDC# 72617-5001-4
Manufactured for:
American Standard Nutraceuticals
Las Vegas, Nevada 89120
allergymix.com 800-959-37961 81163 00005 9
LOT # XXXX
res
-
INGREDIENTS AND APPEARANCE
ALLERGY MIX LAS VEGAS
local remedy las vegas tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72617-5001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALTERNARIA ALTERNATA (UNII: 52B29REC7H) (ALTERNARIA ALTERNATA - UNII:52B29REC7H) ALTERNARIA ALTERNATA 6 [hp_X] PASSALORA FULVA (UNII: HR6H5057CO) (PASSALORA FULVA - UNII:HR6H5057CO) PASSALORA FULVA 6 [hp_X] OLEA EUROPAEA FLOWER (UNII: 498M34P1VZ) (OLEA EUROPAEA FLOWER - UNII:498M34P1VZ) OLEA EUROPAEA FLOWER 6 [hp_X] WHITE MULBERRY (UNII: MN25R0HH5A) (WHITE MULBERRY - UNII:MN25R0HH5A) WHITE MULBERRY 6 [hp_X] JUNIPERUS ASHEI POLLEN (UNII: 544F8MEY0Y) (JUNIPERUS ASHEI POLLEN - UNII:544F8MEY0Y) JUNIPERUS ASHEI POLLEN 6 [hp_X] NERIUM OLEANDER FLOWER (UNII: WO4WVF1WVM) (NERIUM OLEANDER FLOWER - UNII:WO4WVF1WVM) NERIUM OLEANDER FLOWER 6 [hp_X] JUNIPERUS SCOPULORUM POLLEN (UNII: 0G82TT8ZFY) (JUNIPERUS SCOPULORUM POLLEN - UNII:0G82TT8ZFY) JUNIPERUS SCOPULORUM POLLEN 6 [hp_X] USTILAGO MAYDIS (UNII: 4K7Z7K7SWG) (USTILAGO MAYDIS - UNII:4K7Z7K7SWG) USTILAGO MAYDIS 6 [hp_X] Inactive Ingredients Ingredient Name Strength DEXTROSE (UNII: IY9XDZ35W2) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72617-5001-4 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/23/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/23/2019 Labeler - ASN (012050067) Registrant - ASN (012050067)