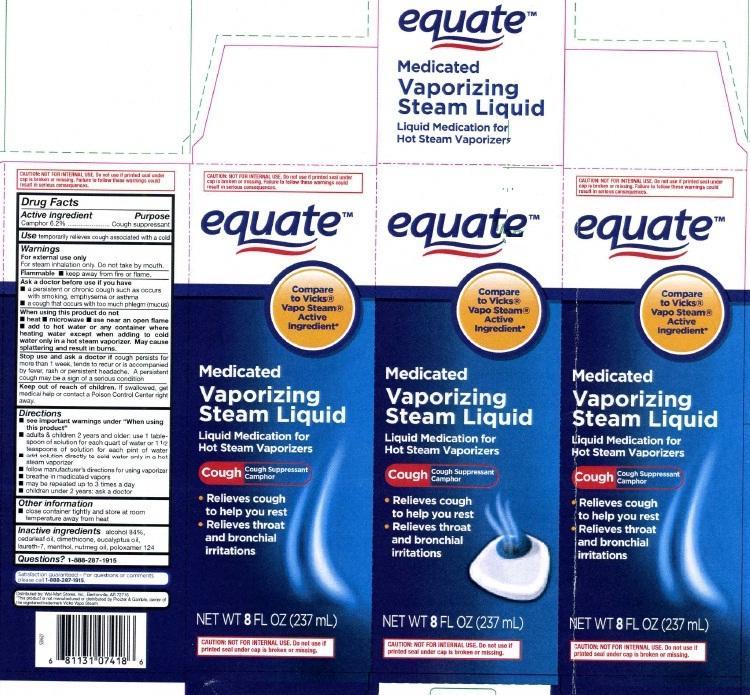
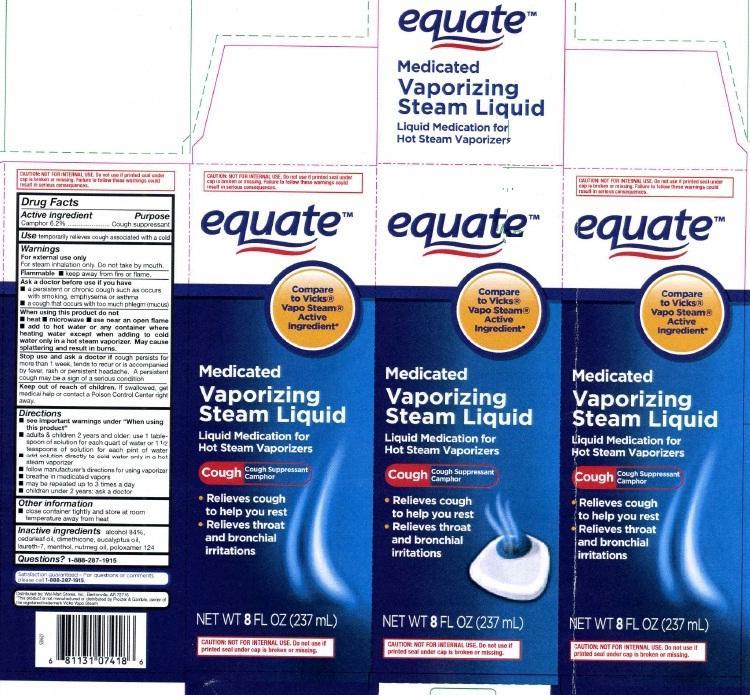
Label: VAPORIZING- camphor inhalant
- NDC Code(s): 49035-099-34
- Packager: Wal-Mart Stores, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- safety information
- Active Ingredient
- Purpose
- Use
- Warnings
- Flammable
- Ask a doctor before use if you have
- When using this product do not
- Stop use and ask a doctor
- Keep out of reach of children
-
Directions
- see important warnings under "When using this product"
- adults & children 2 years and older: use 1 tablespoon of solution for each quart of water or 1 1/2 teaspoons of sulution for each pint of water
- add solution directly to cold water only in a hot steam vaporizer
- follow manufacturer's directions for using vaporizer
- breath in medicated vapors
- may be repeated up to 3 times a day
- children under 2 years: ask a doctor
- Other information
- Inactive ingredients
- Questions?
- guarantee claim
- Disclaimer
- Adverse reactions section
-
principal display panel
equate
Compare to Vicks
Vapo Steam
Active Ingredient
Medicated
Vaporizing
Steam Liquid
Liquid Medication for Hot Steam Vaporizers
Cough Cough Suppressant Camphor
Relieves cough to help you rest
Relieves throat and bronchial irritation
NET WT 8 FL OZ (237 mL)CAUTION NOT FOR INTERNAL USE. Do not use if printed seal under cap is broken or missing.
-
INGREDIENTS AND APPEARANCE
VAPORIZING
camphor inhalantProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-099 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 62 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) DIMETHICONE (UNII: 92RU3N3Y1O) EUCALYPTUS OIL (UNII: 2R04ONI662) LAURETH-7 (UNII: Z95S6G8201) MENTHOL (UNII: L7T10EIP3A) NUTMEG OIL (UNII: Z1CLM48948) POLOXAMER 124 (UNII: 1S66E28KXA) CEDAR LEAF OIL (UNII: BJ169U4NLG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-099-34 1 in 1 CARTON 03/07/2014 1 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 03/07/2014 Labeler - Wal-Mart Stores, Inc (051957769) Registrant - Consumer Product Partners, LLC (119091520) Establishment Name Address ID/FEI Business Operations Consumer Product Partners, LLC 119091520 manufacture(49035-099) Establishment Name Address ID/FEI Business Operations Consumer Product Partners, LLC 119091514 manufacture(49035-099)