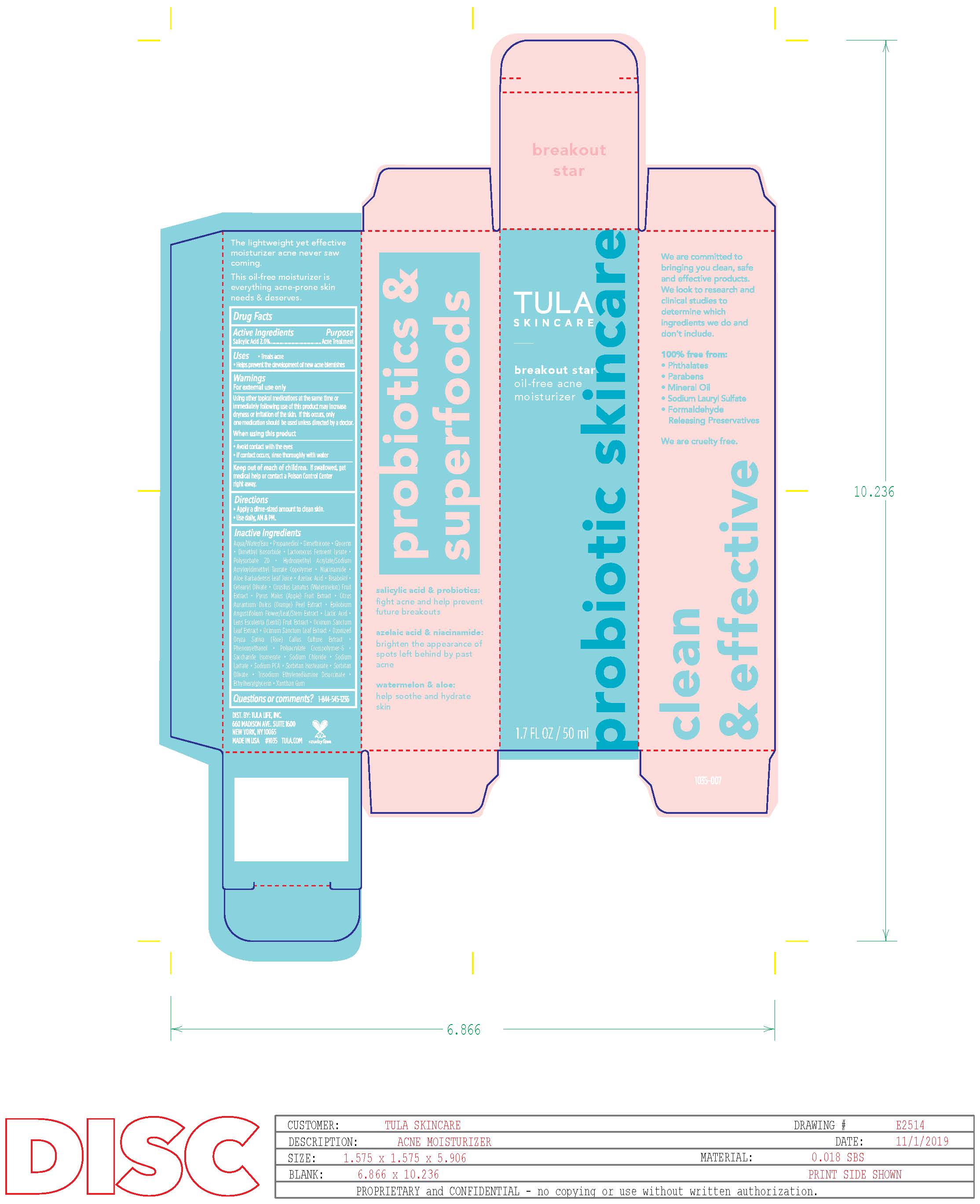
Label: TULA BREAKOUT STAR OIL-FREE ACNE MOISTURIZER liquid
- NDC Code(s): 72296-020-15, 72296-020-50
- Packager: Tula Life LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only
Using other topical medications at the same time or
immediately following use of this product may increase
dryness or irritation of the skin. If this occurs, only one
medication should be used unless directed by a doctor.When using this product
• avoid contact with the eyes
• if contact occurs, rinse thoroughly with water - Keep out of reach of children
-
Directions
• clean the skin thoroughly before applying this product
• cover the entire affected area with a thin layer one to three
times daily
• because excessive drying of the skin may occur, start with
one application daily, then gradually increase to two or three
times daily if needed or as directed by a doctor
• if bothersome dryness or peeling occurs, reduce application
to once a day or every other day -
Inactive ingredients
Aqua/Water/Eau, Propanediol, Dimethicone, Glycerin, Dimethyl Isosorbide, Lactococcus Ferment Lysate, Polysorbate 20, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Niacinamide, Aloe Barbadensis Leaf Juice, Azelaic Acid, Bisabolol, Cetearyl Olivate, Cirusllus Lanatus (Watermelon) Fruit Extract, Pyrus Malus (Apple) Fruit Extract, Citrus Aurantium Dulcis (Orange) Peel Extract, Epilobium
Angustifolium Flower/Leaf/Stem Extract, Lactic Acid, Lens Esculenta (Lentil) Fruit Extract, Ocimum Sanctum Leaf Extract, Ozonized Oryza Sativa (Rice) Callus Culture Extract, Phenoxyethanol, Polyacrylate Crosspolymer-6, Saccharide Isomerate, Sodium Chloride, Sodium Lactate, Sodium PCA, Sorbitan Isostearate, Sorbitan Olivate, Trisodium Ethylenediamine Disuccinate, Ethylhexylglycerin, Xanthan Gum - Questions or Comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TULA BREAKOUT STAR OIL-FREE ACNE MOISTURIZER
tula breakout star oil-free acne moisturizer liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72296-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) POLYSORBATE 20 (UNII: 7T1F30V5YH) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) NIACINAMIDE (UNII: 25X51I8RD4) ALOE VERA LEAF (UNII: ZY81Z83H0X) AZELAIC ACID (UNII: F2VW3D43YT) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) CETEARYL OLIVATE (UNII: 58B69Q84JO) CITRULLUS LANATUS WHOLE (UNII: 3J5I6254YO) CITRUS AURANTIUM FLOWER (UNII: O730ZX2Z83) EPILOBIUM ANGUSTIFOLIUM WHOLE (UNII: C278QS9YBT) LACTIC ACID (UNII: 33X04XA5AT) ORYZA SATIVA WHOLE (UNII: 84IVV0906Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) SACCHARIDE ISOMERATE (UNII: W8K377W98I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) SORBITAN OLIVATE (UNII: MDL271E3GR) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) XANTHAN GUM (UNII: TTV12P4NEE) APPLE (UNII: B423VGH5S9) WATERMELON (UNII: 231473QB6R) LENTIL (UNII: 6O38V6B52O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72296-020-50 1 in 1 CARTON 03/21/2024 1 50 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:72296-020-15 1 in 1 CARTON 03/21/2024 2 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/21/2024 Labeler - Tula Life LLC (080051358)