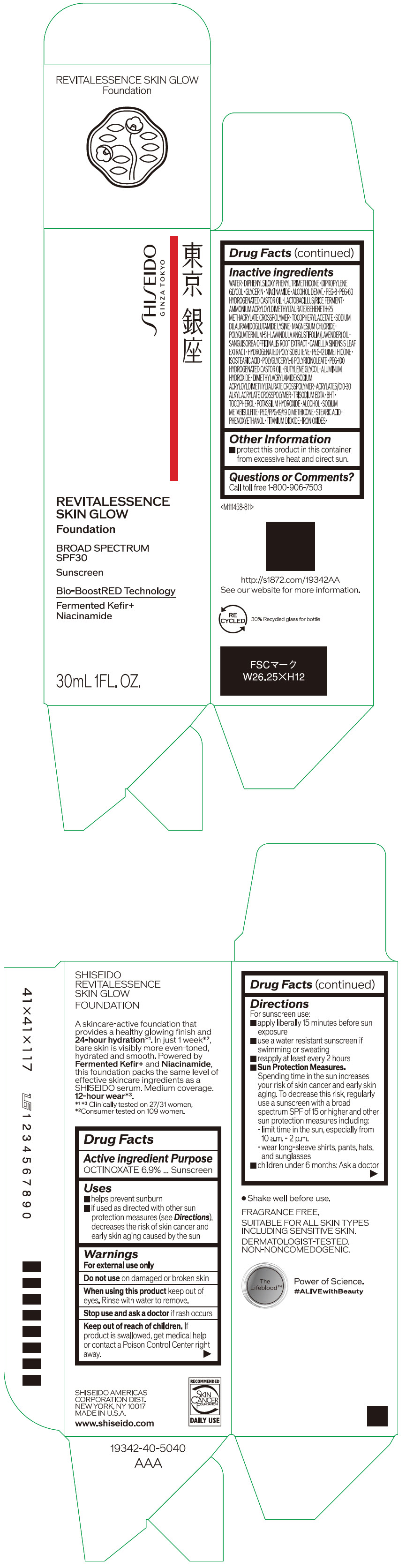
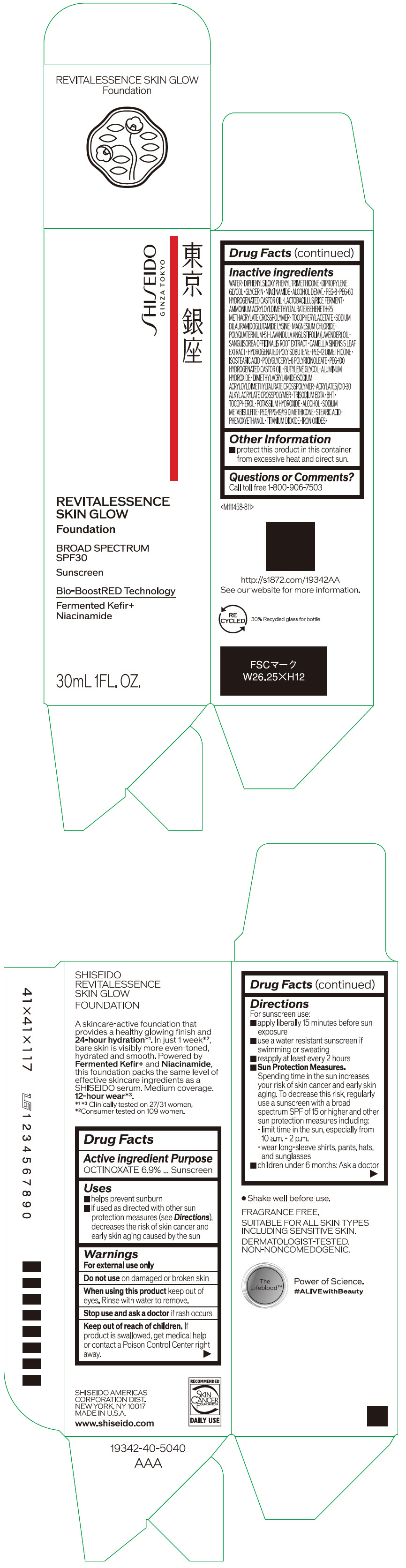
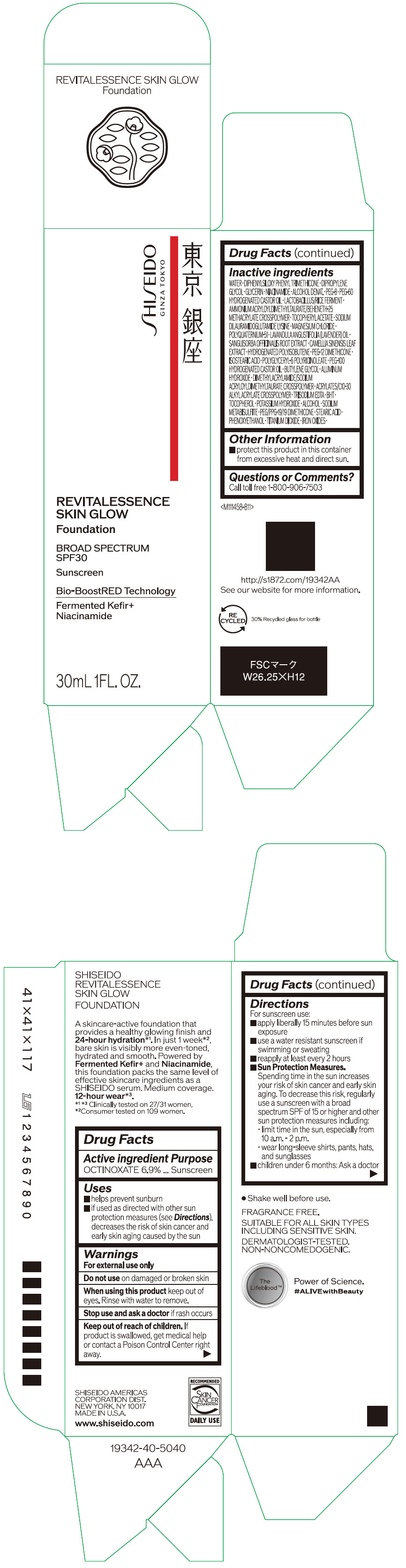
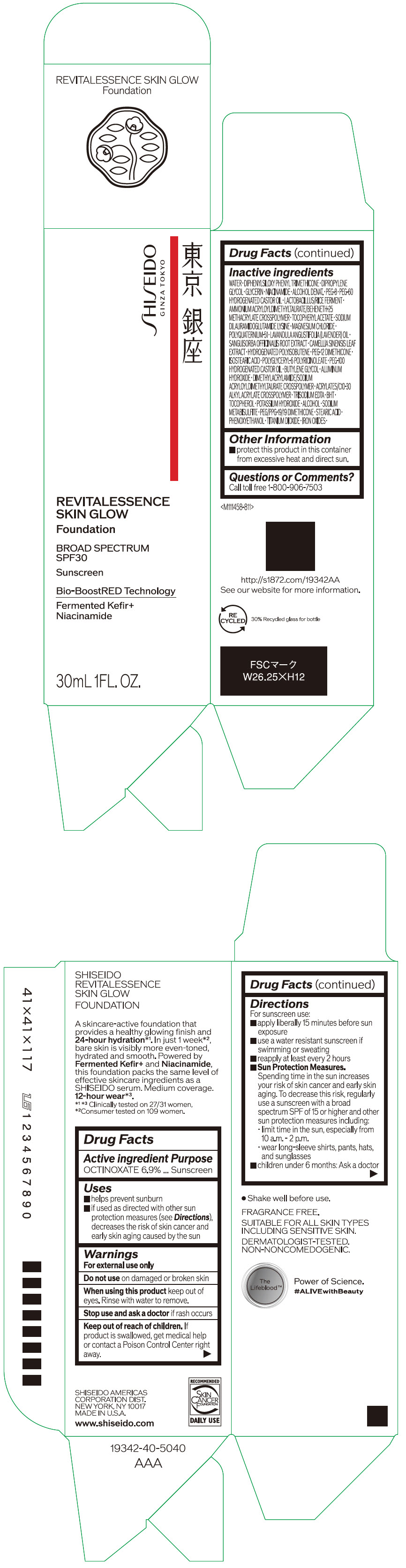
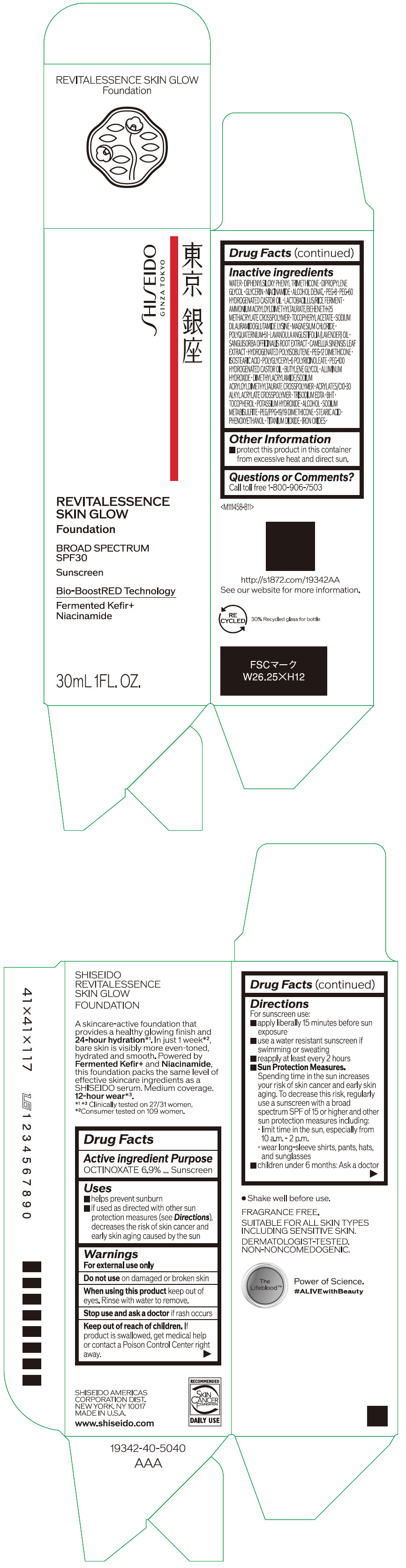
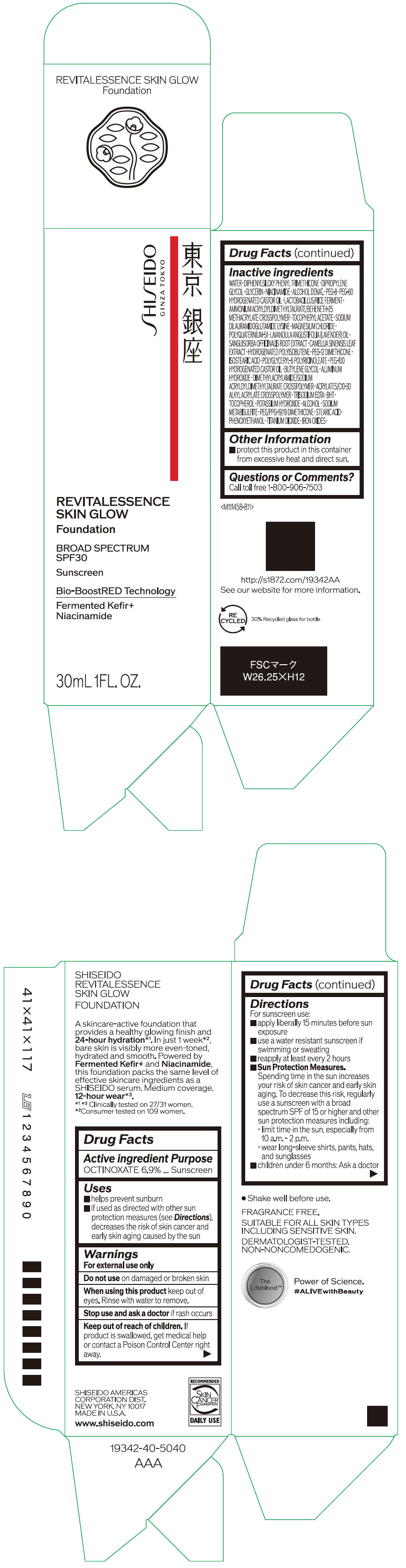
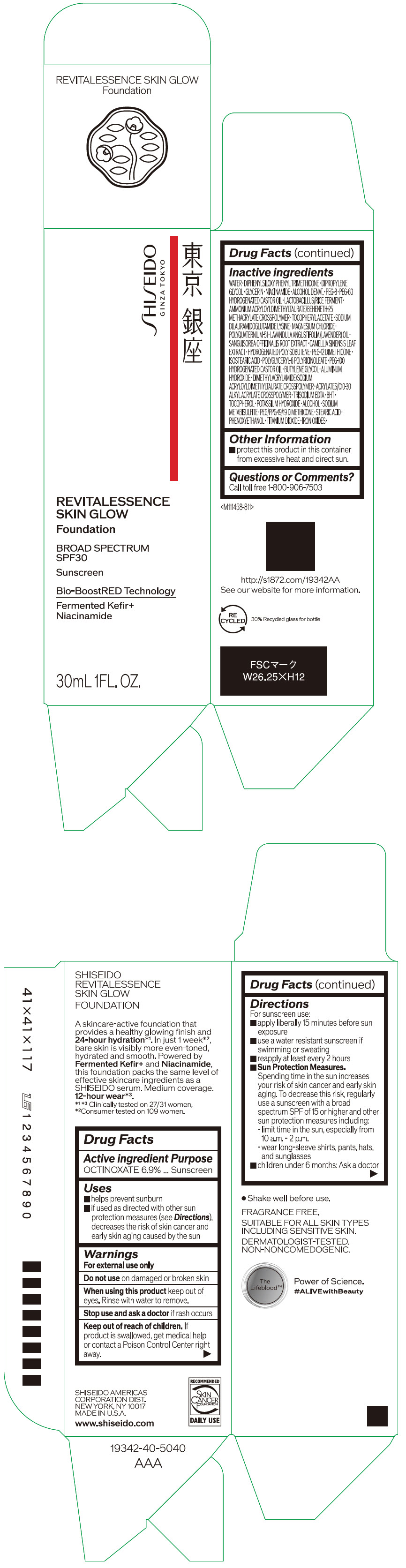
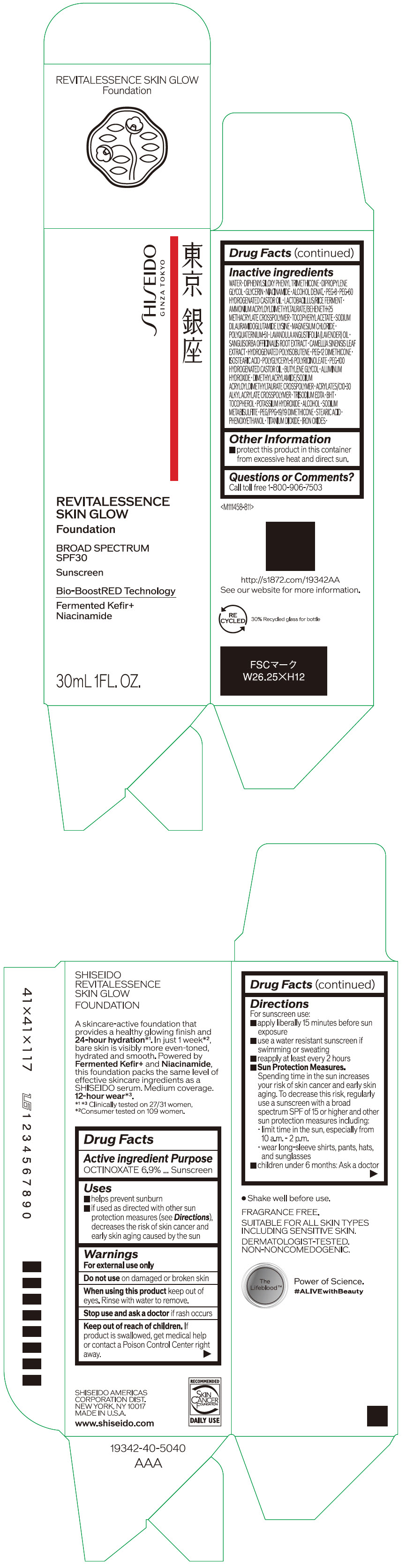
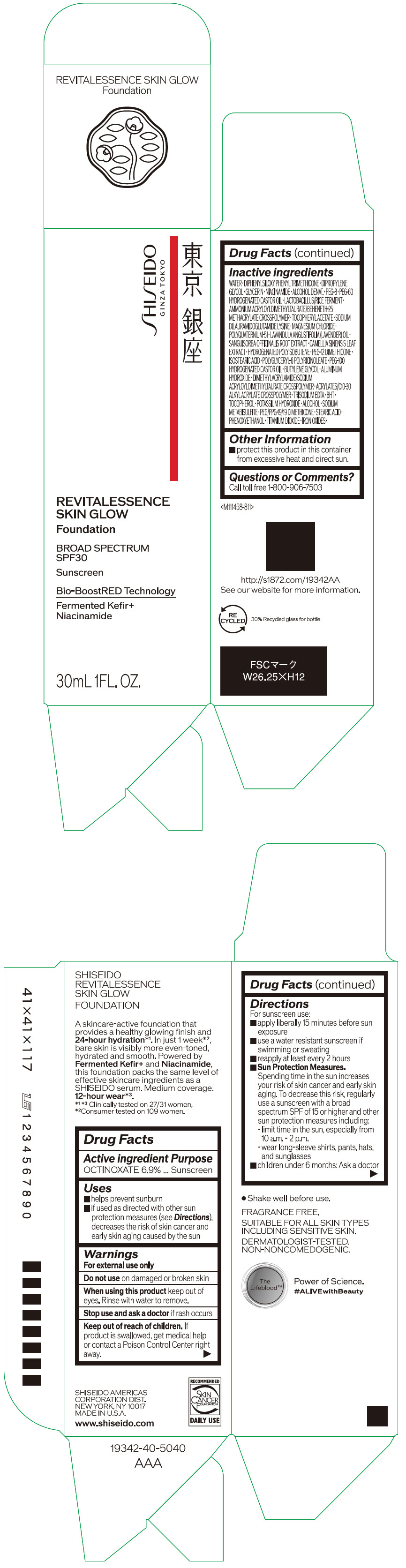
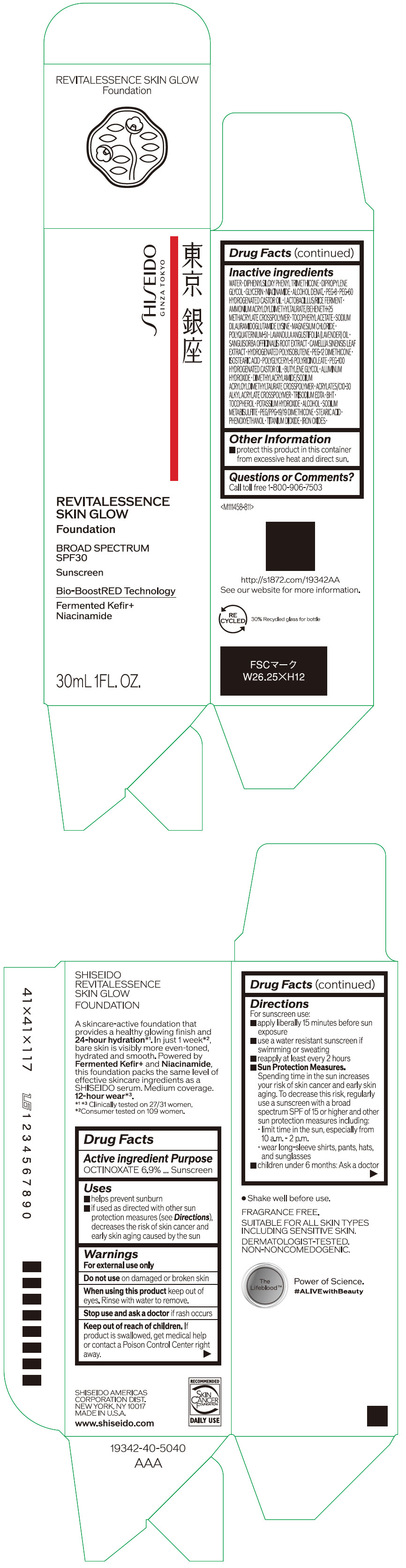
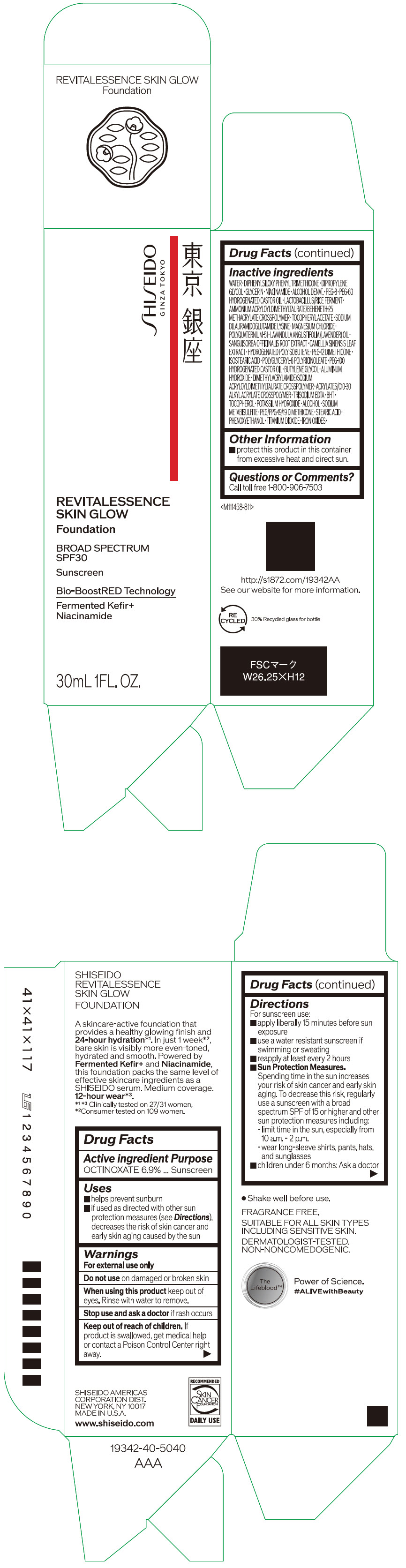
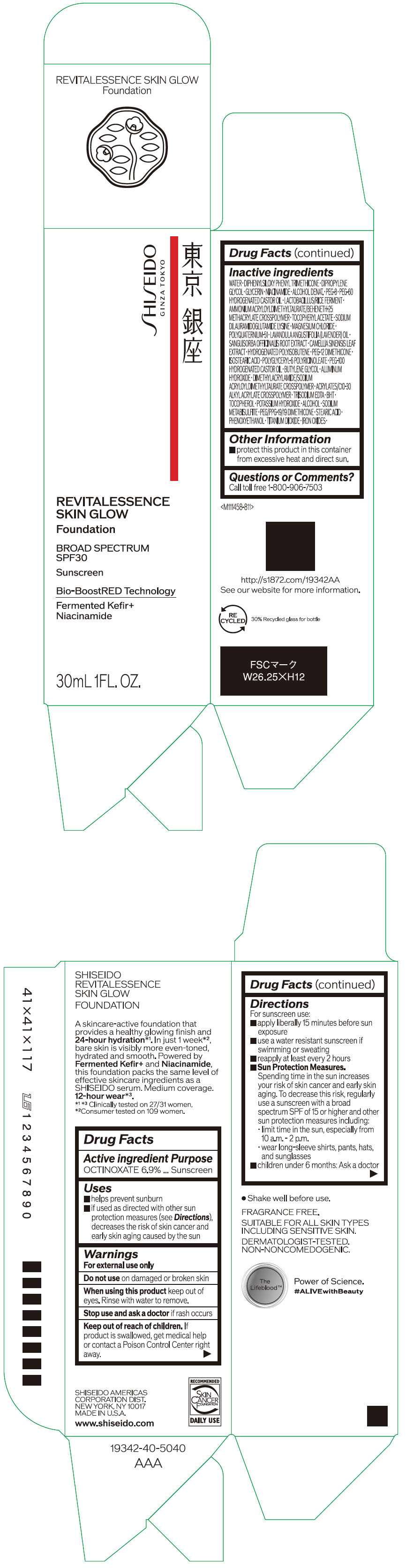
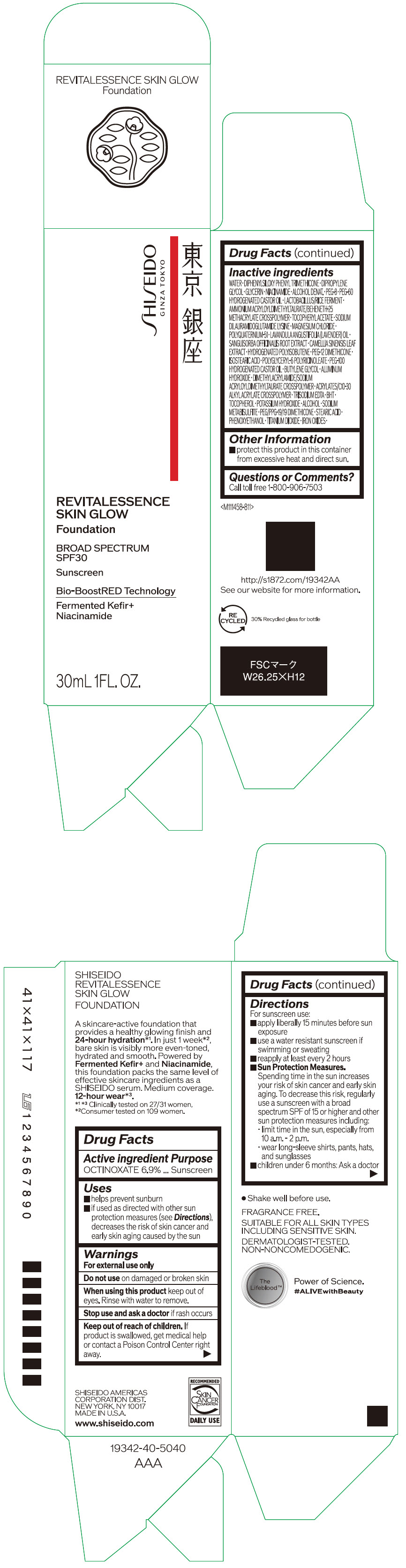
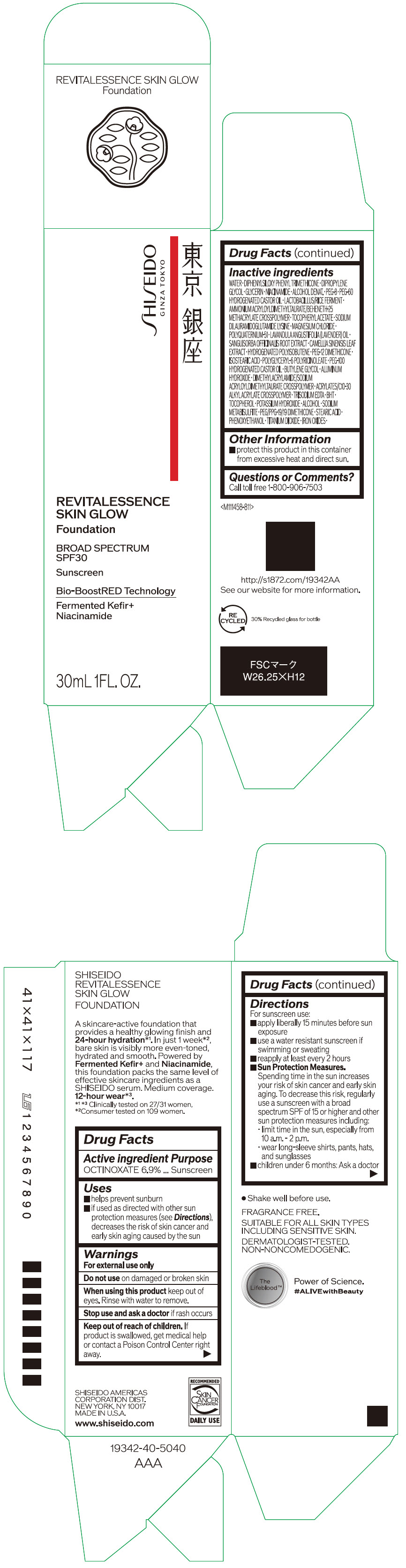
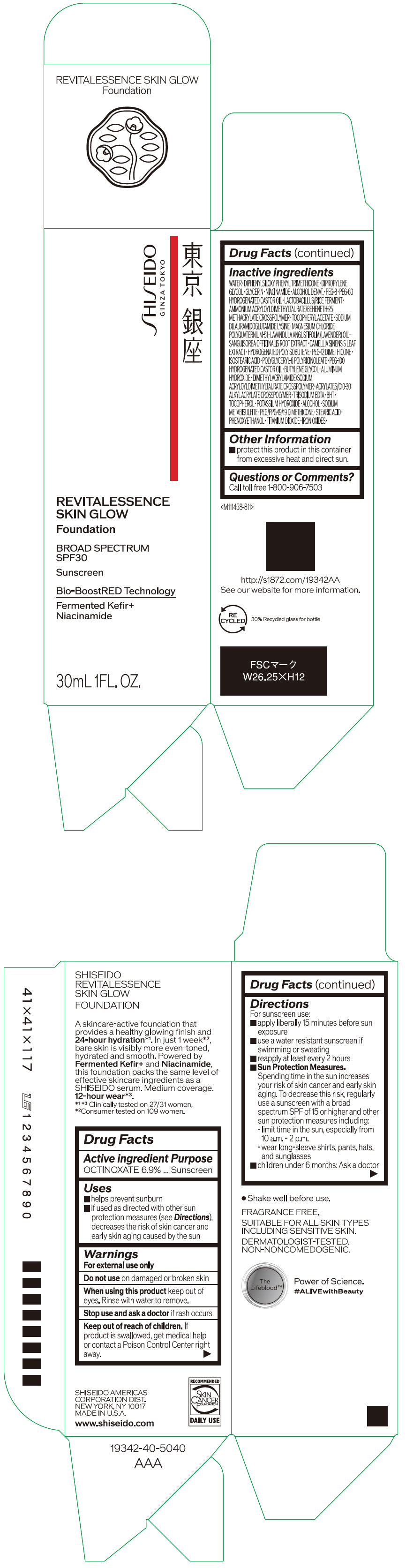
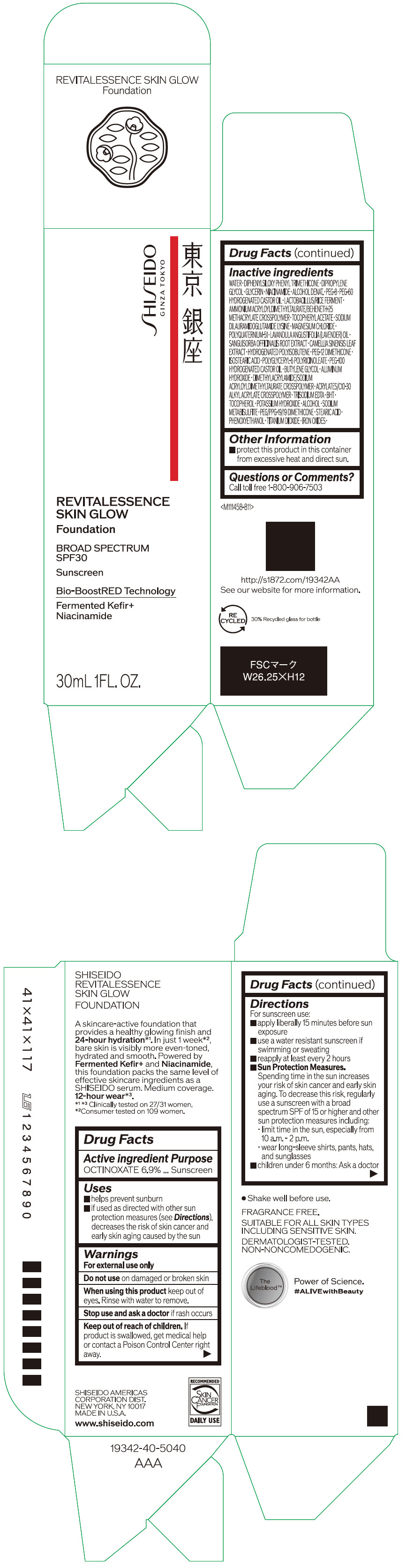
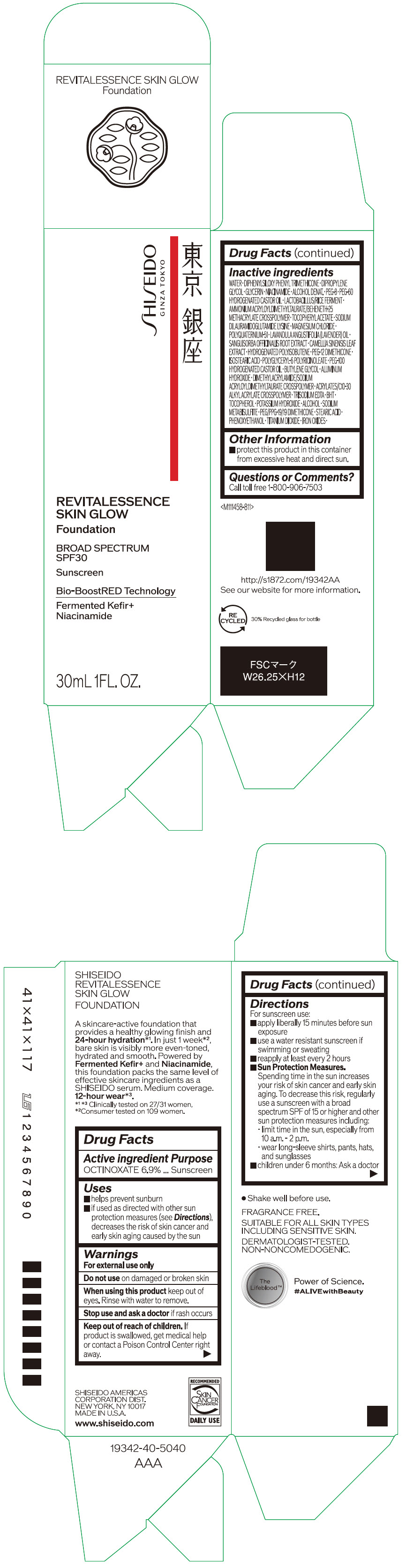
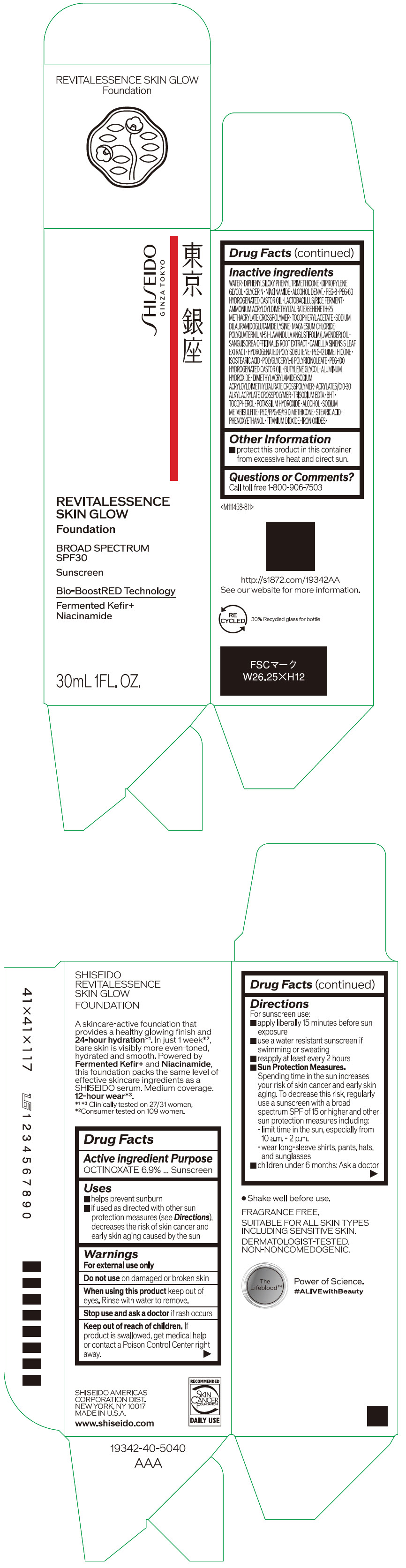
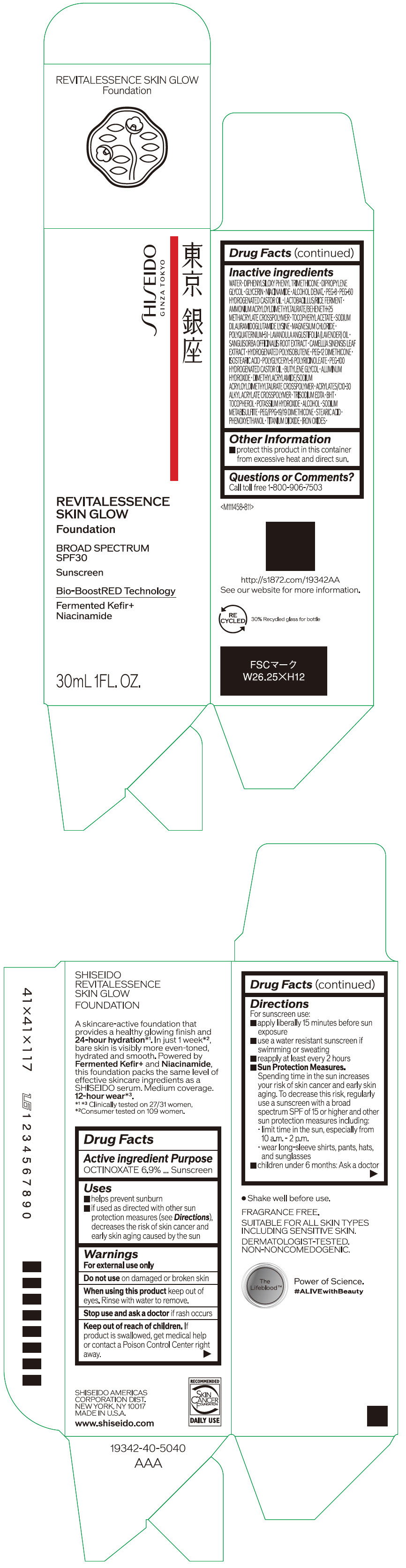
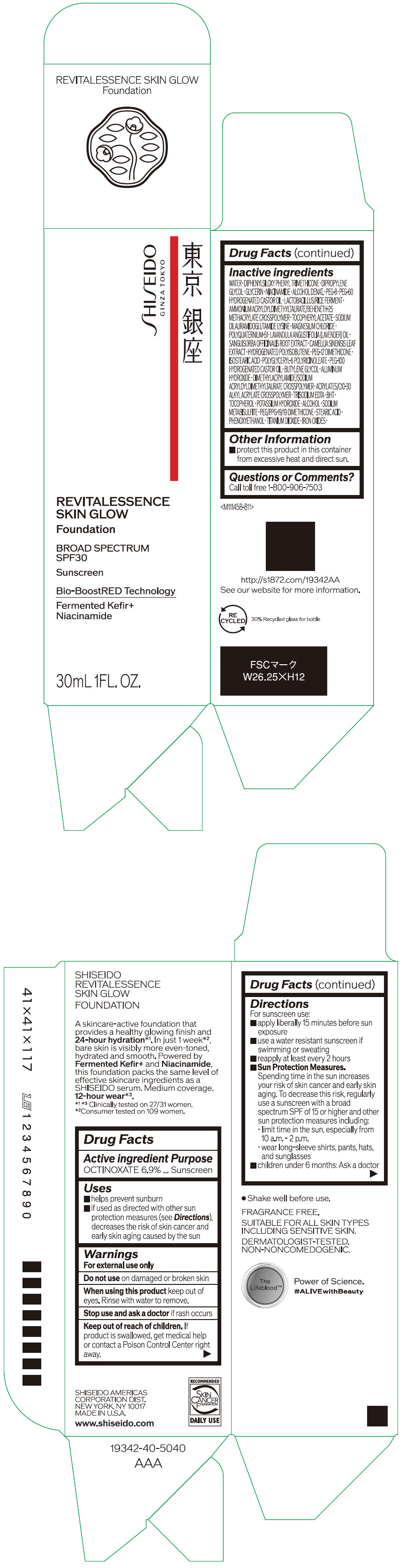
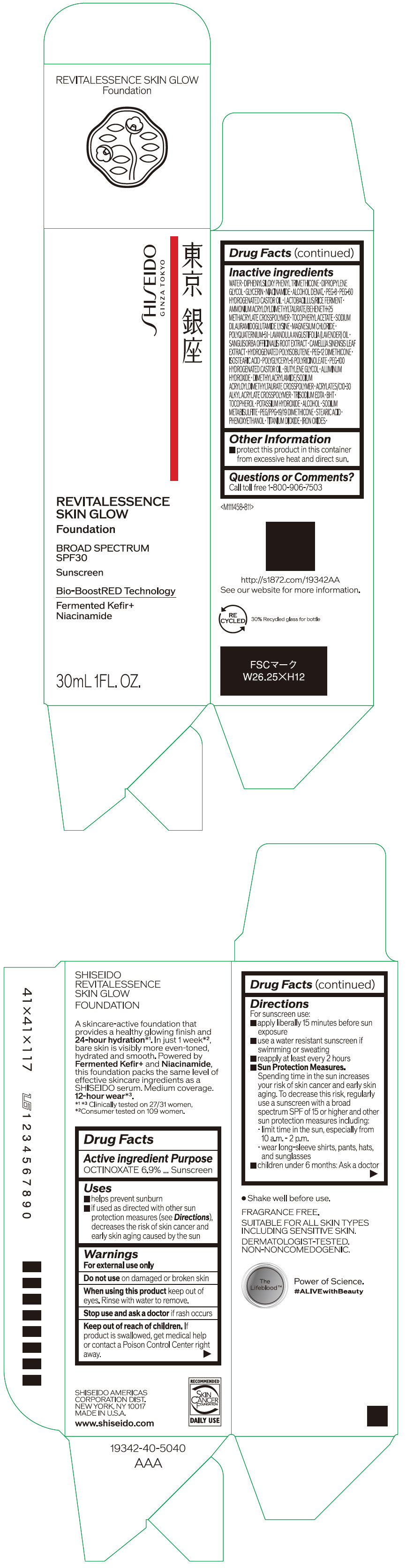
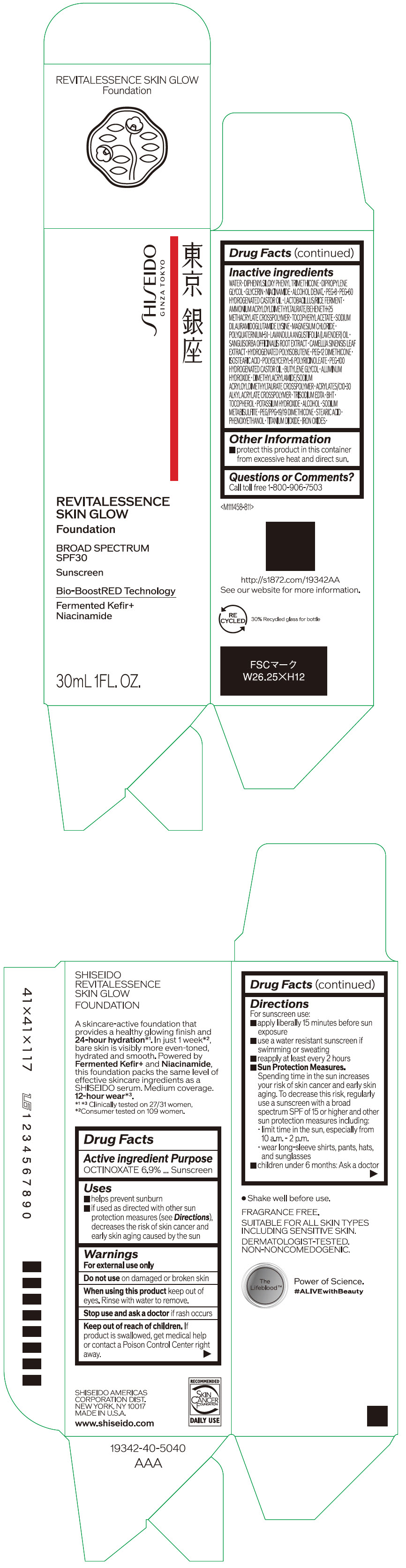
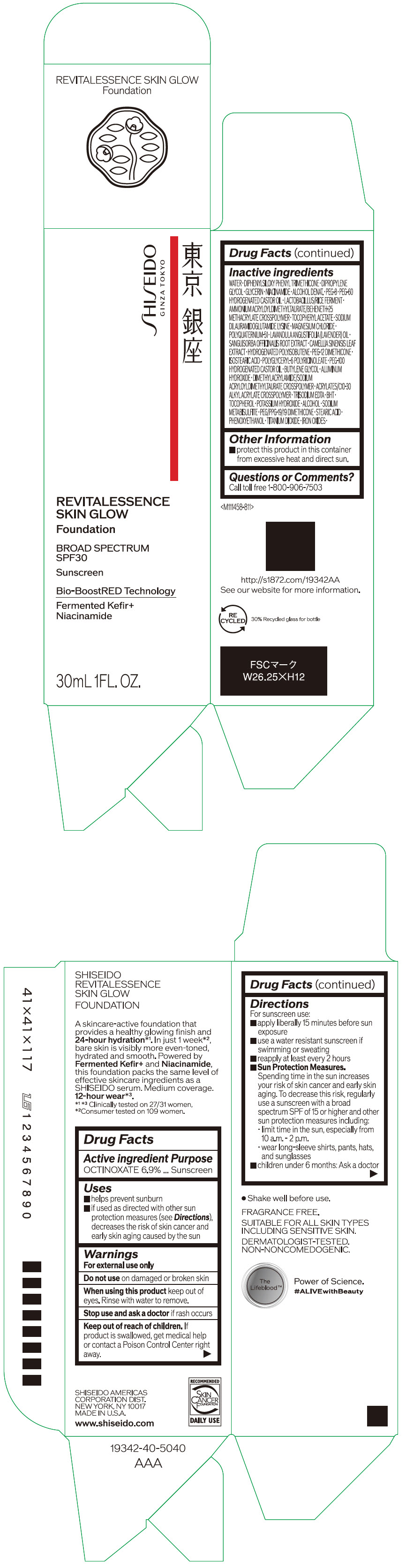
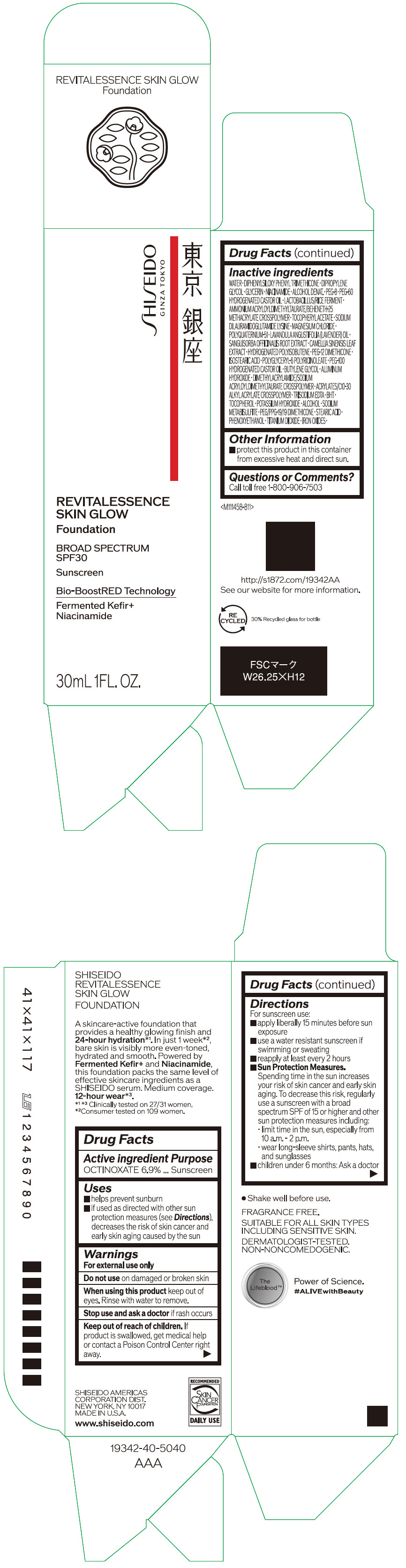
Label: SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 110- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 120- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 130- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 140- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUND .......OUNDATION 510- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 520- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 530- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 540- octinoxate emulsion
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 550- octinoxate emulsion
-
NDC Code(s):
58411-859-40,
58411-860-40,
58411-861-40,
58411-862-40, view more58411-863-40, 58411-864-40, 58411-865-40, 58411-866-40, 58411-867-40, 58411-868-40, 58411-869-40, 58411-870-40, 58411-871-40, 58411-872-40, 58411-873-40, 58411-874-40, 58411-875-40, 58411-876-40, 58411-877-40, 58411-878-40, 58411-879-40, 58411-880-40, 58411-881-40, 58411-882-40, 58411-883-40, 58411-884-40, 58411-885-40, 58411-886-40, 58411-887-40
- Packager: SHISEIDO AMERICAS CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
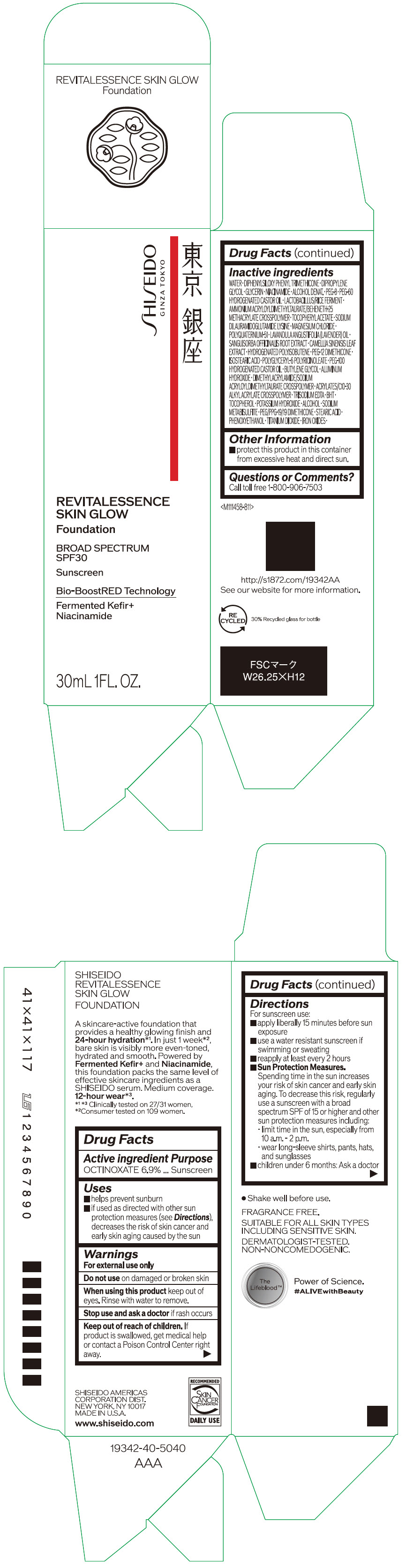
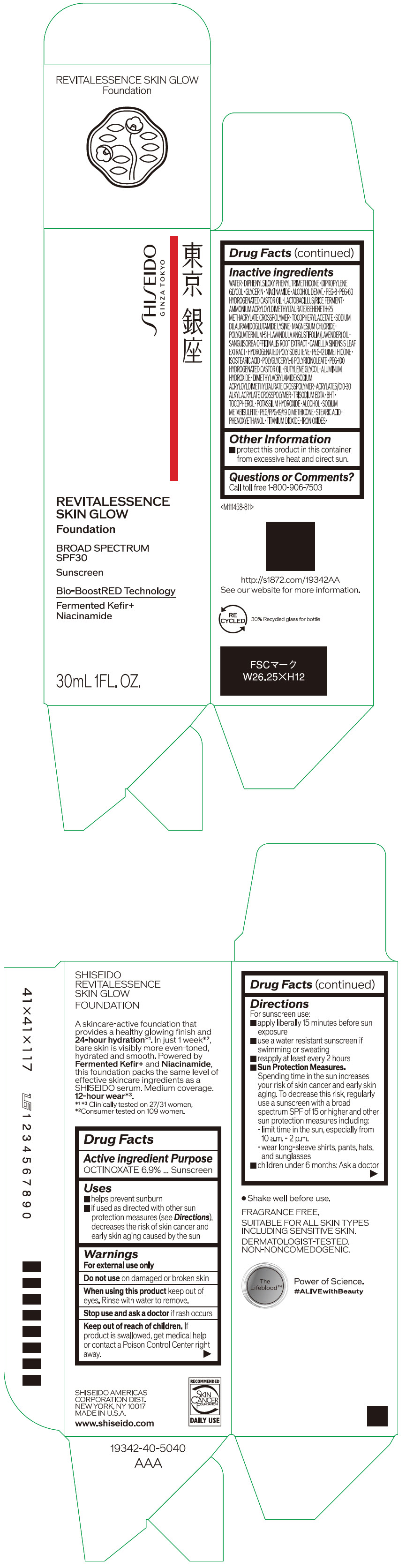
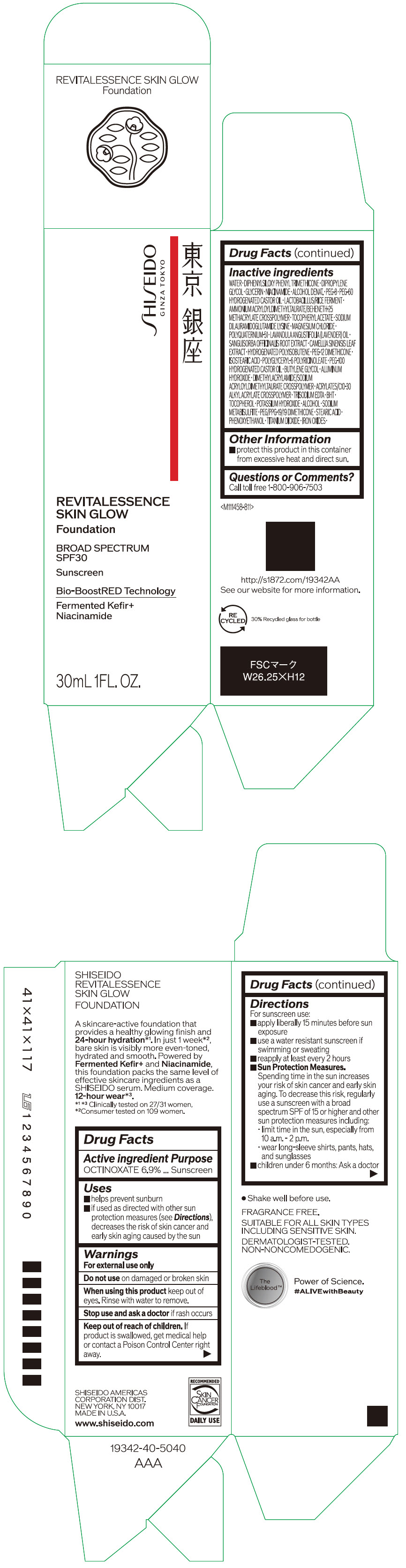
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
WATER・DIPHENYLSILOXY PHENYL TRIMETHICONE・DIPROPYLENE GLYCOL・GLYCERIN・NIACINAMIDE・ALCOHOL DENAT.・PEG-8・PEG-60 HYDROGENATED CASTOR OIL・LACTOBACILLUS/RICE FERMENT・AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER・TOCOPHERYL ACETATE・SODIUM DILAURAMIDOGLUTAMIDE LYSINE・MAGNESIUM CHLORIDE・POLYQUATERNIUM-51・LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL・SANGUISORBA OFFICINALIS ROOT EXTRACT・CAMELLIA SINENSIS LEAF EXTRACT・HYDROGENATED POLYISOBUTENE・PEG-12 DIMETHICONE・ISOSTEARIC ACID・POLYGLYCERYL-6 POLYRICINOLEATE・PEG-100 HYDROGENATED CASTOR OIL・BUTYLENE GLYCOL・ALUMINUM HYDROXIDE・DIMETHYLACRYLAMIDE/SODIUM ACRYLOYLDIMETHYLTAURATE CROSSPOLYMER・ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER・TRISODIUM EDTA・BHT・TOCOPHEROL・POTASSIUM HYDROXIDE・ALCOHOL・SODIUM METABISULFITE・PEG/PPG-19/19 DIMETHICONE・STEARIC ACID・PHENOXYETHANOL・TITANIUM DIOXIDE・IRON OXIDES・
- Other information
- Questions or comments?
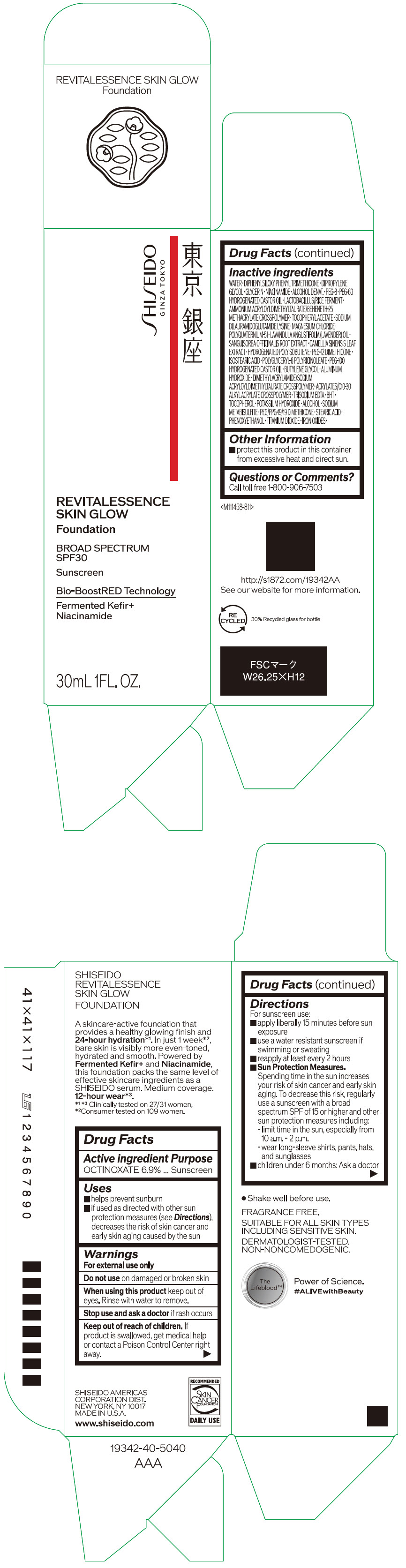
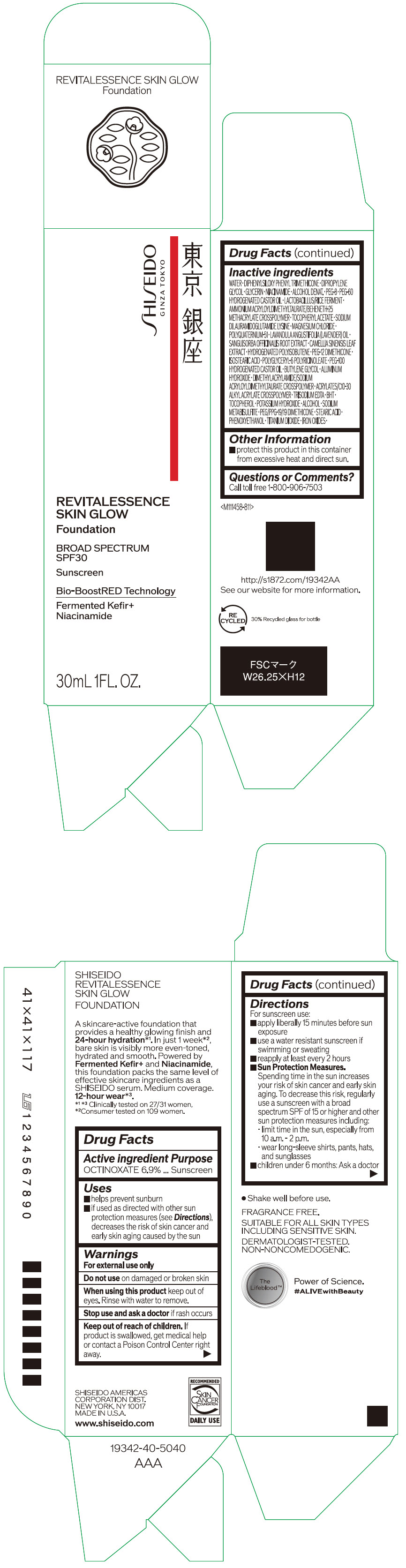
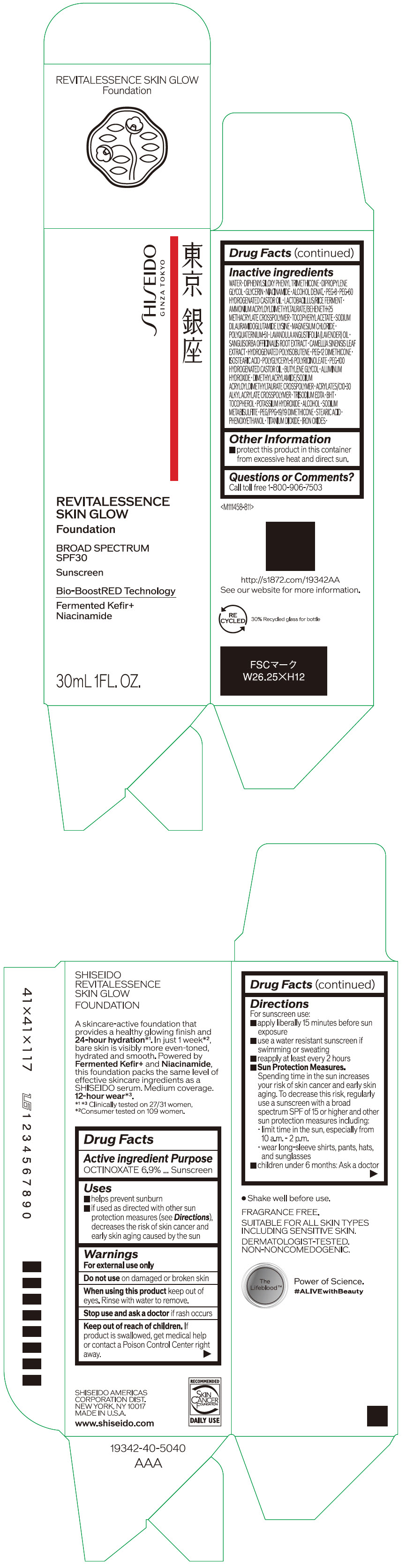
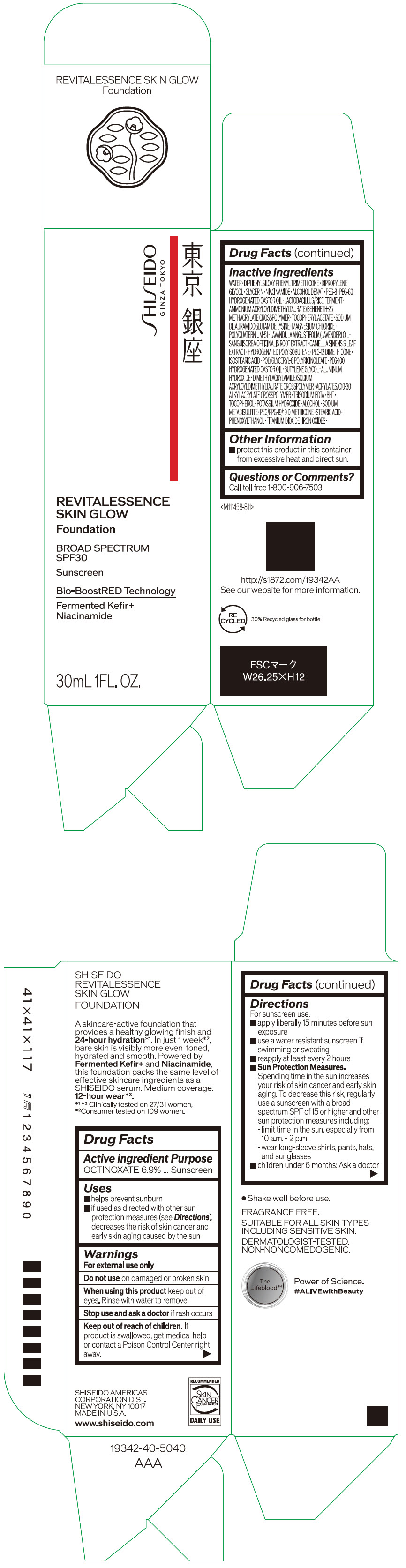
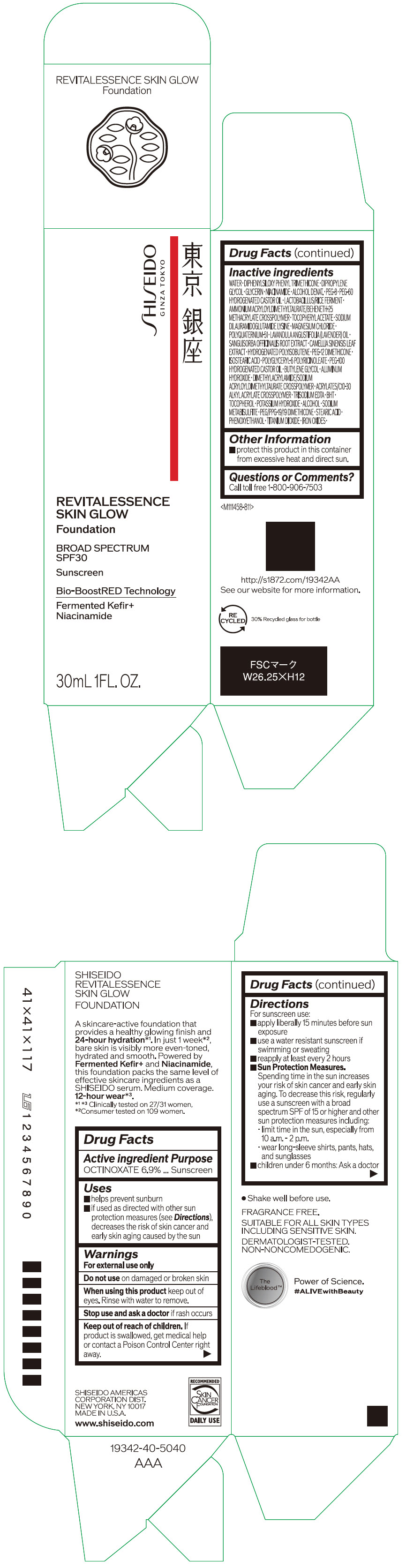
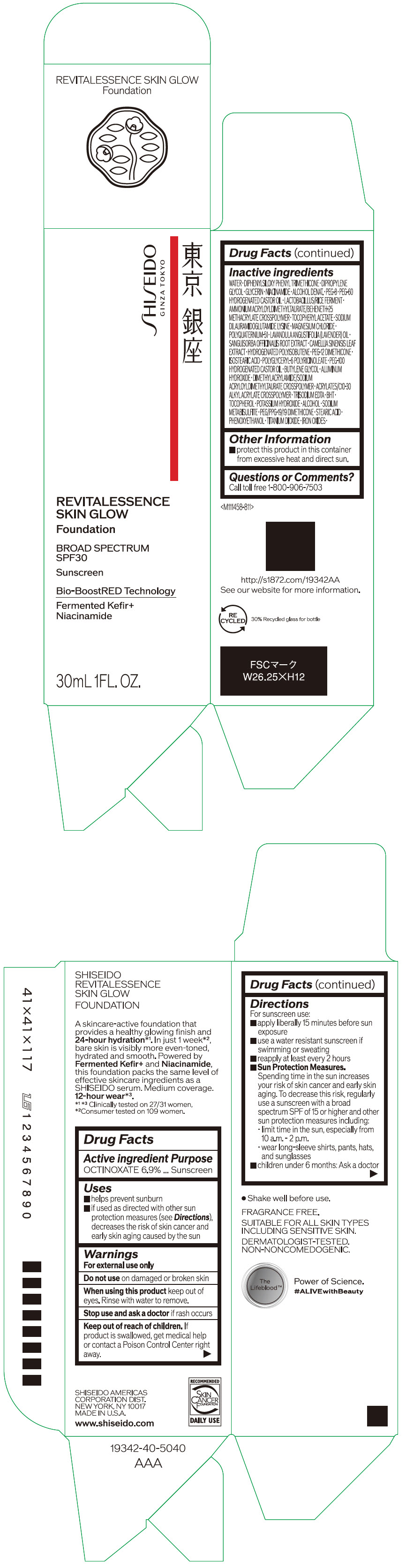
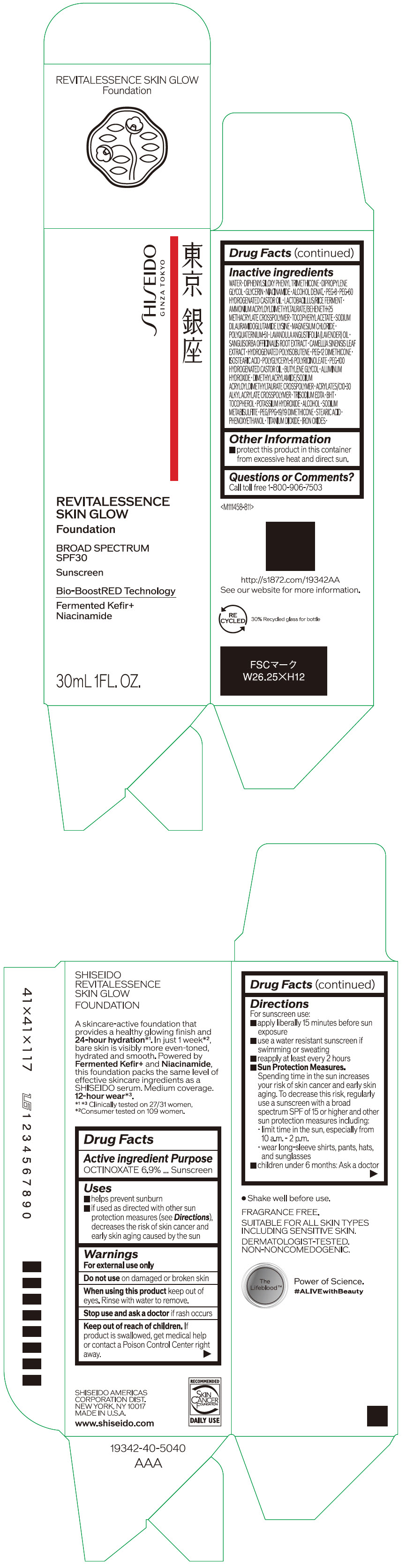
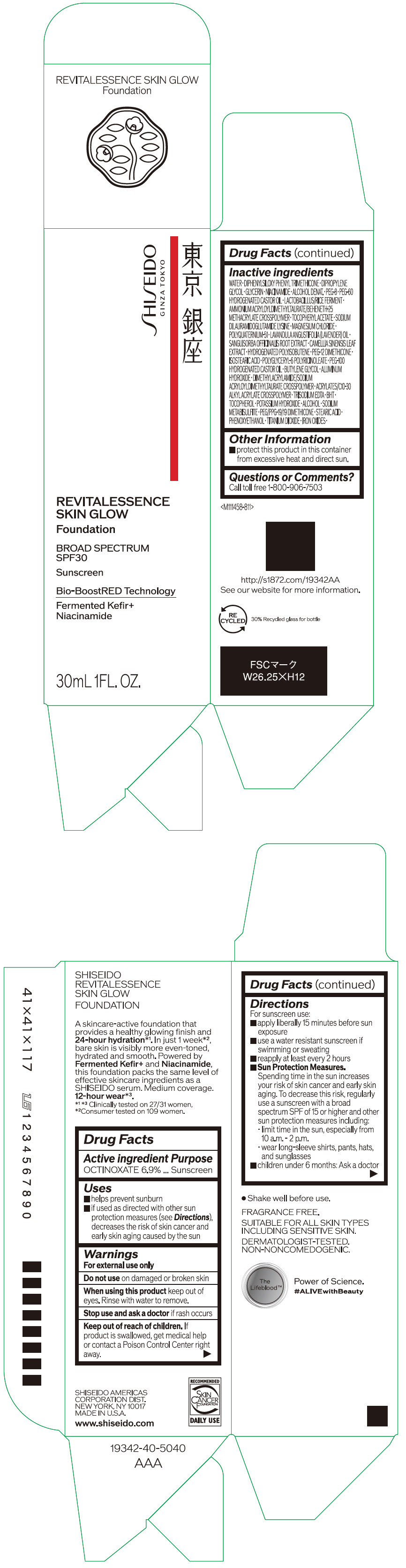
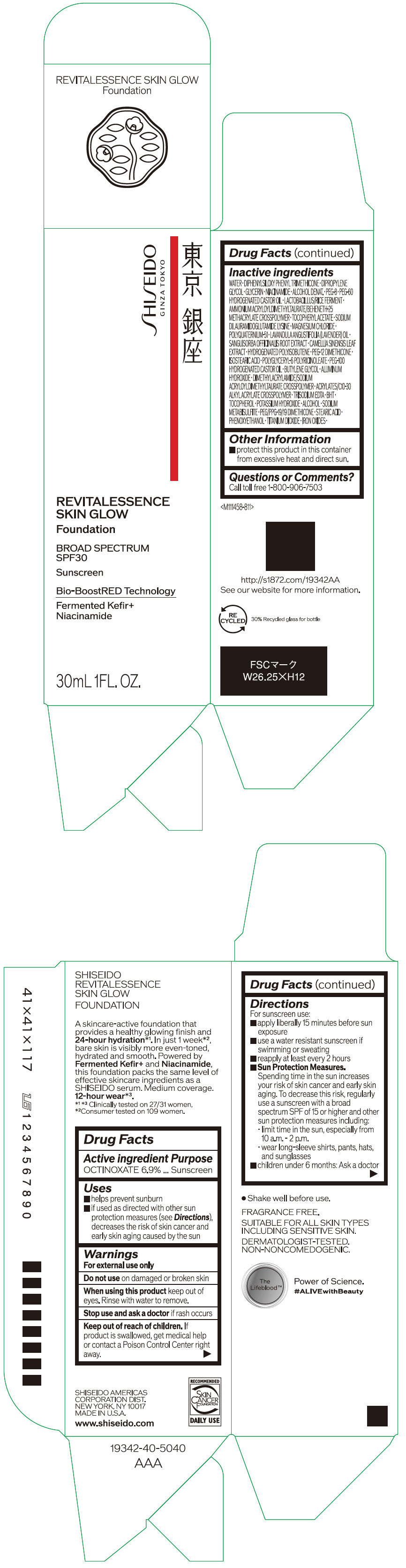
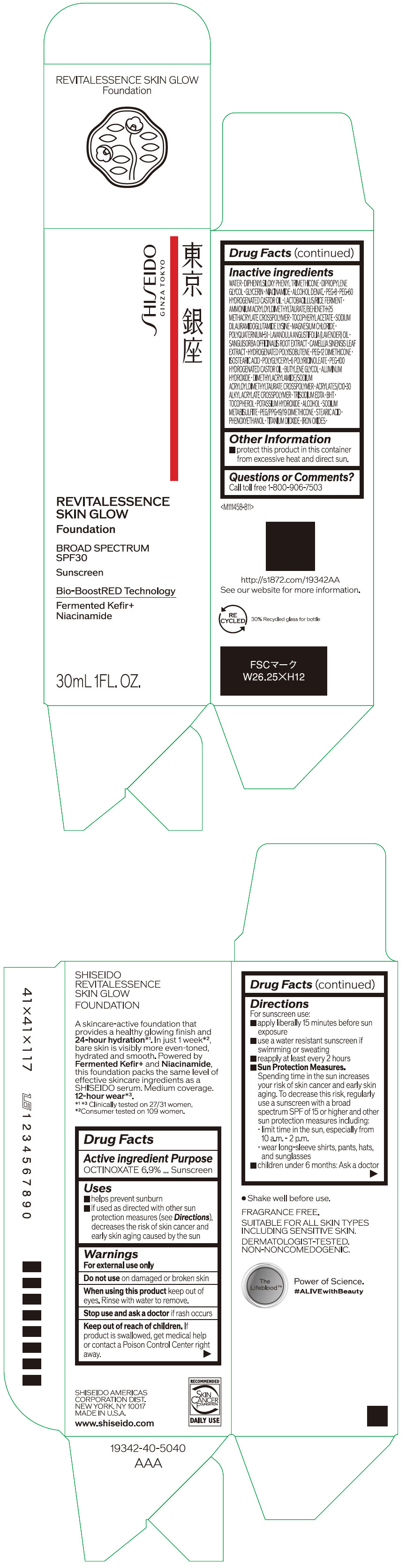
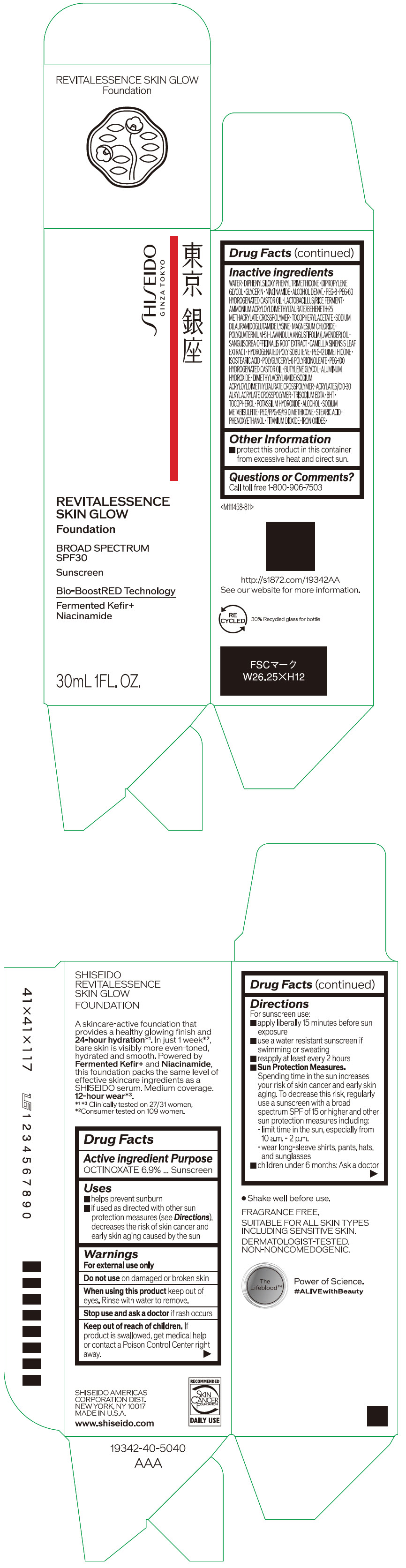
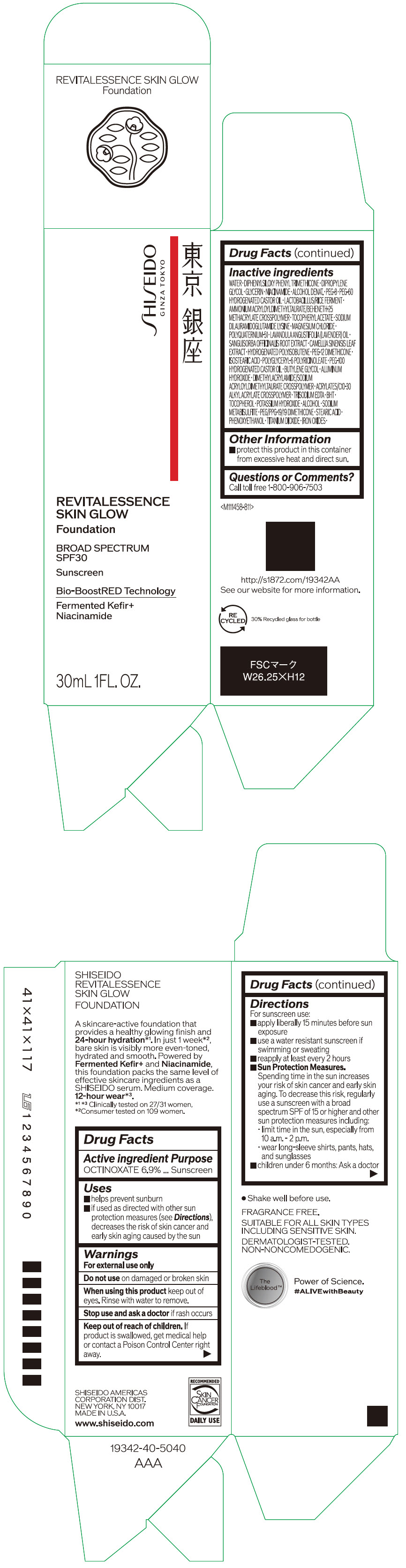
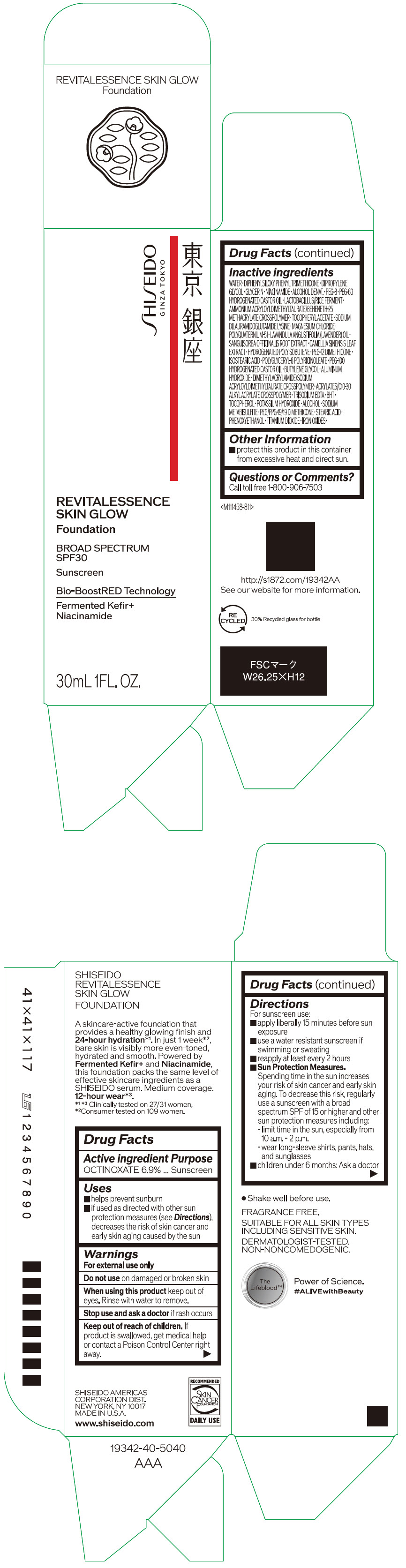
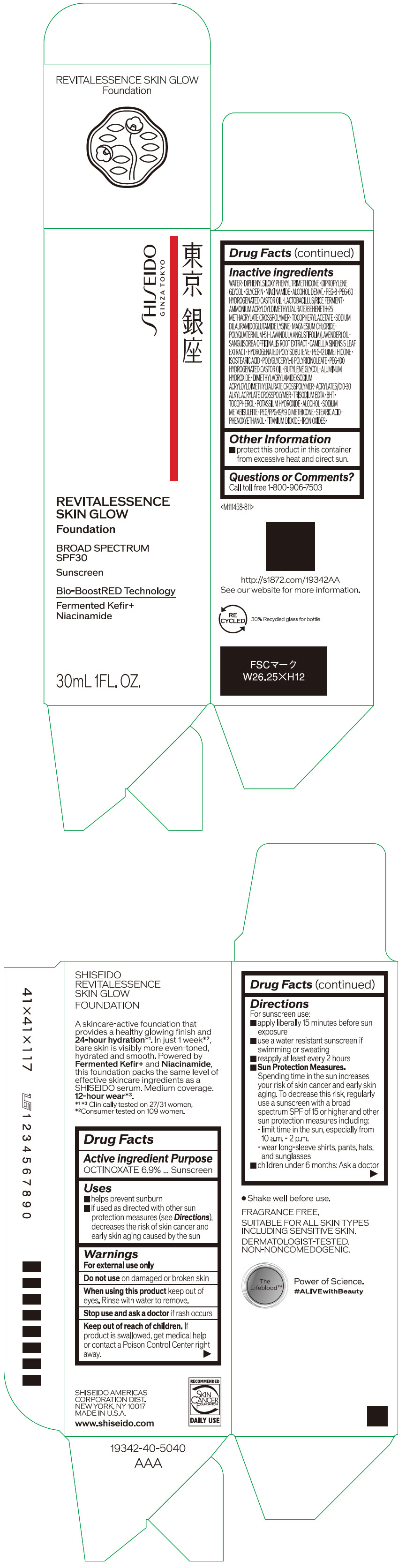
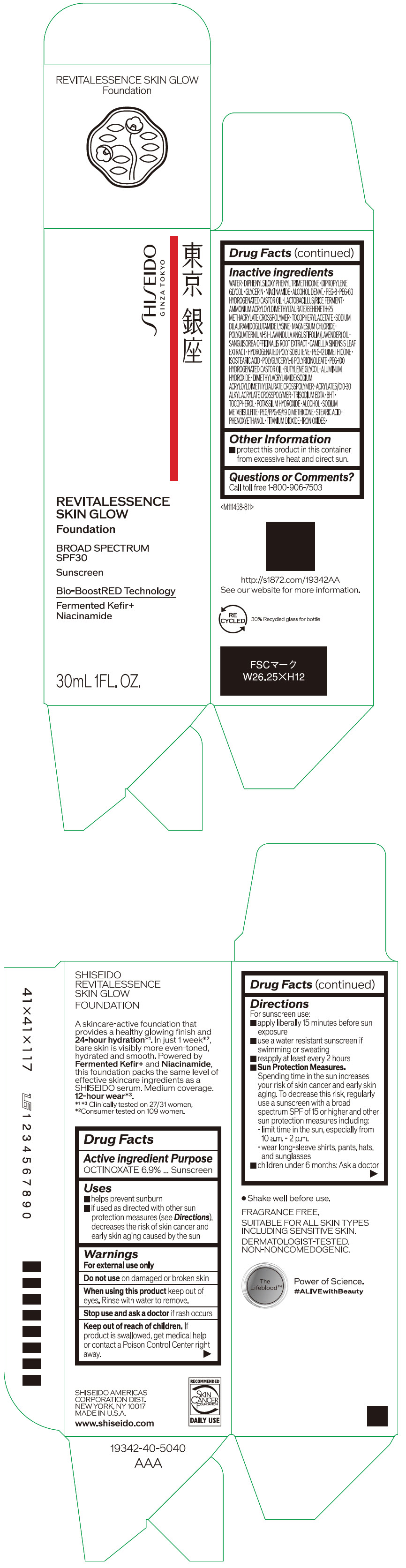
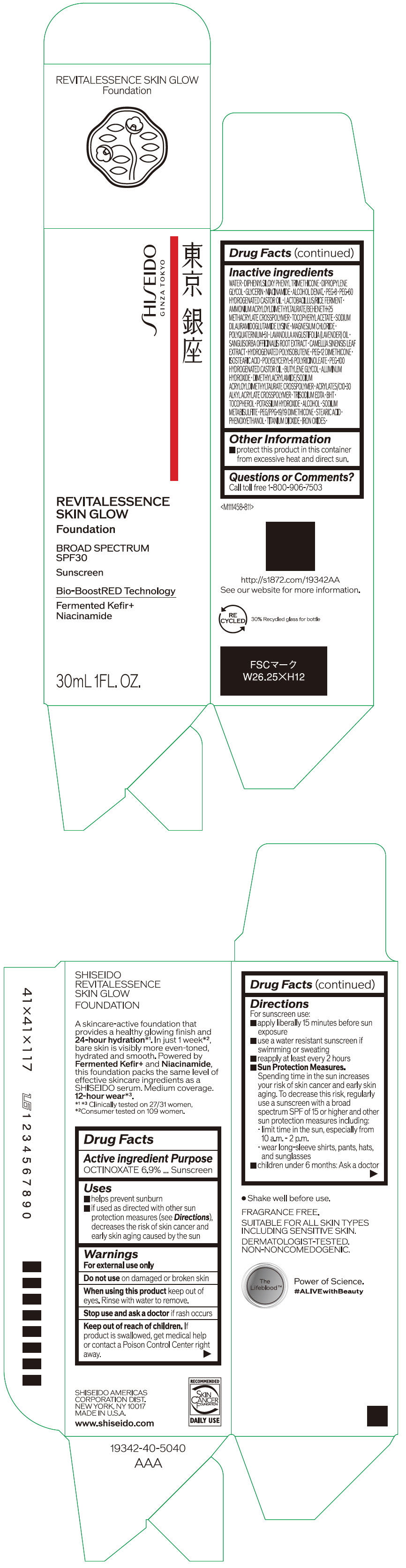
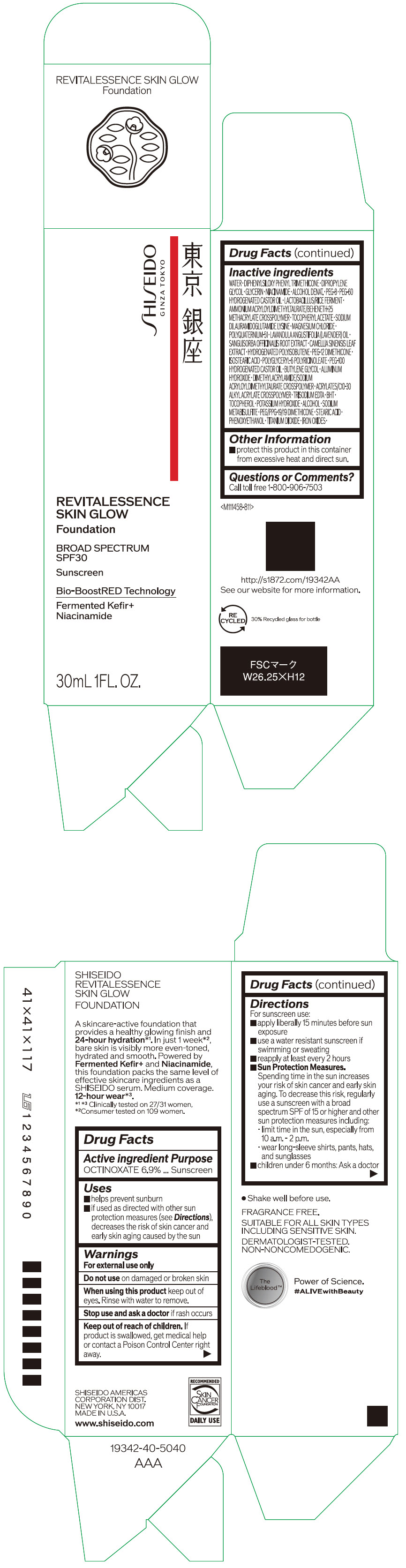
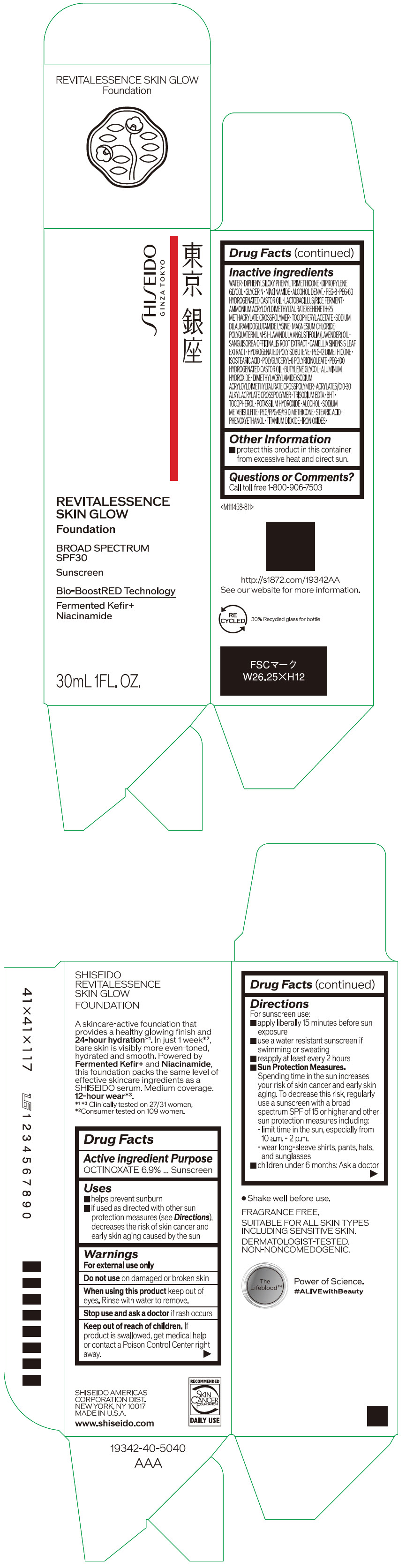
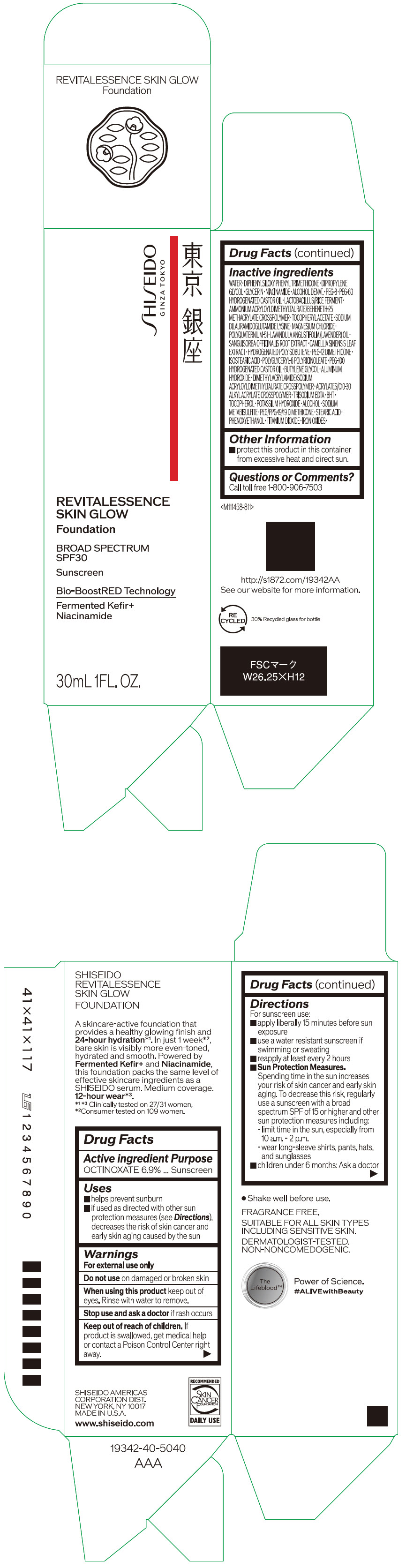
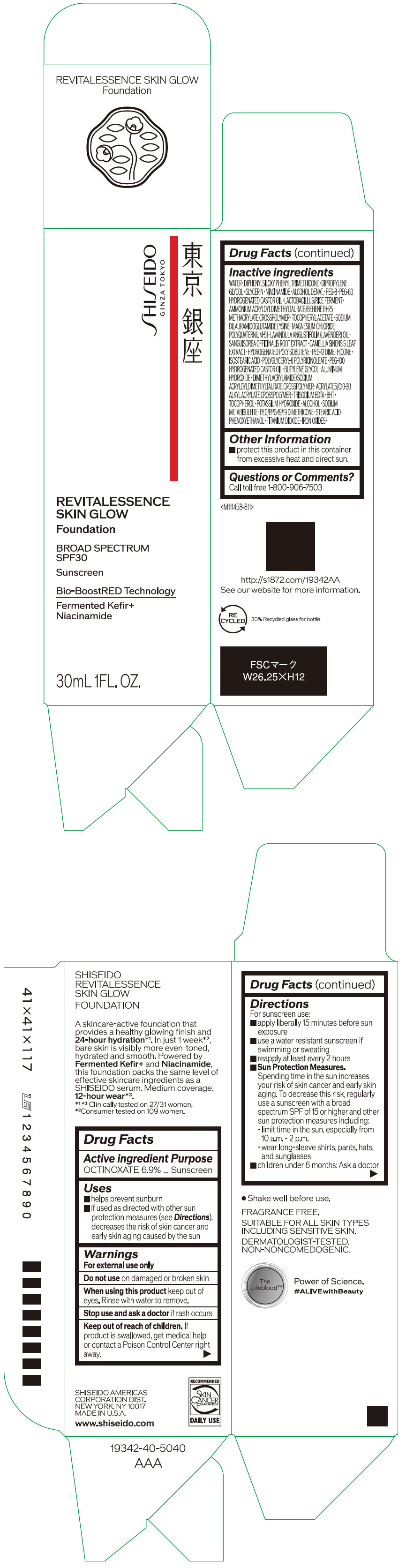
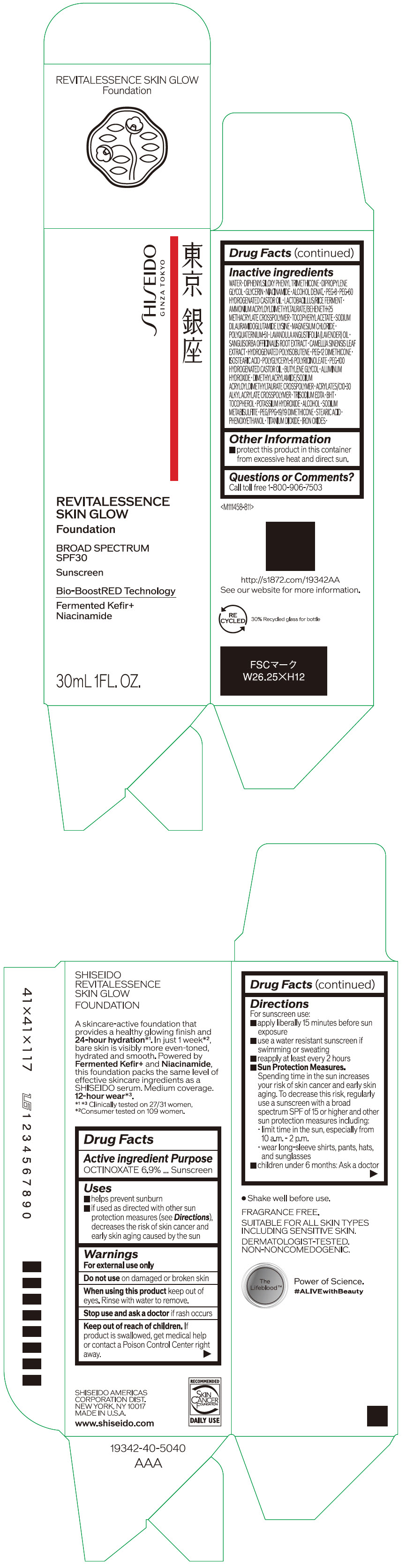
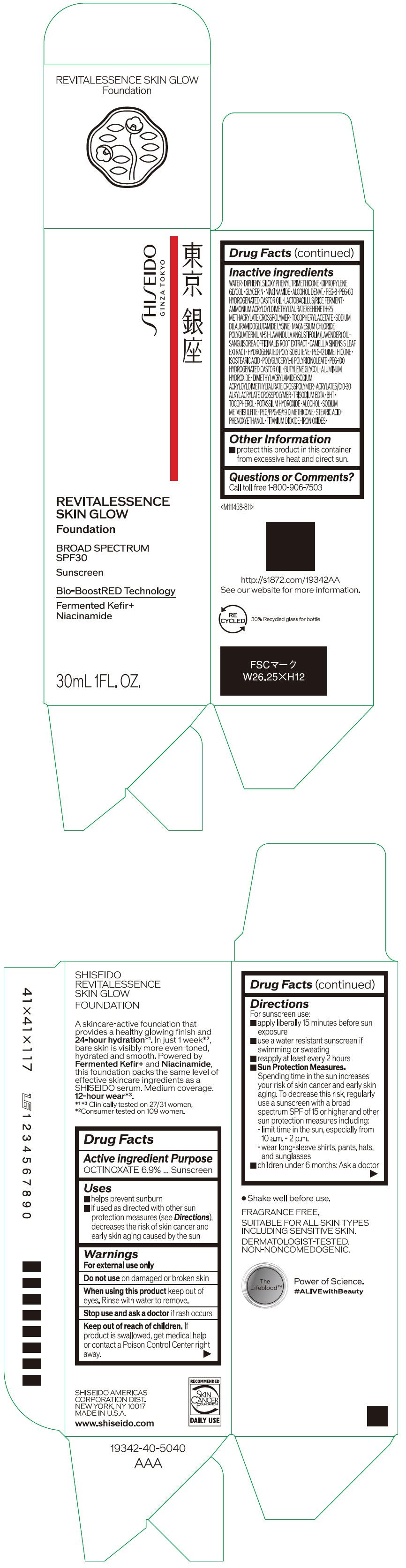
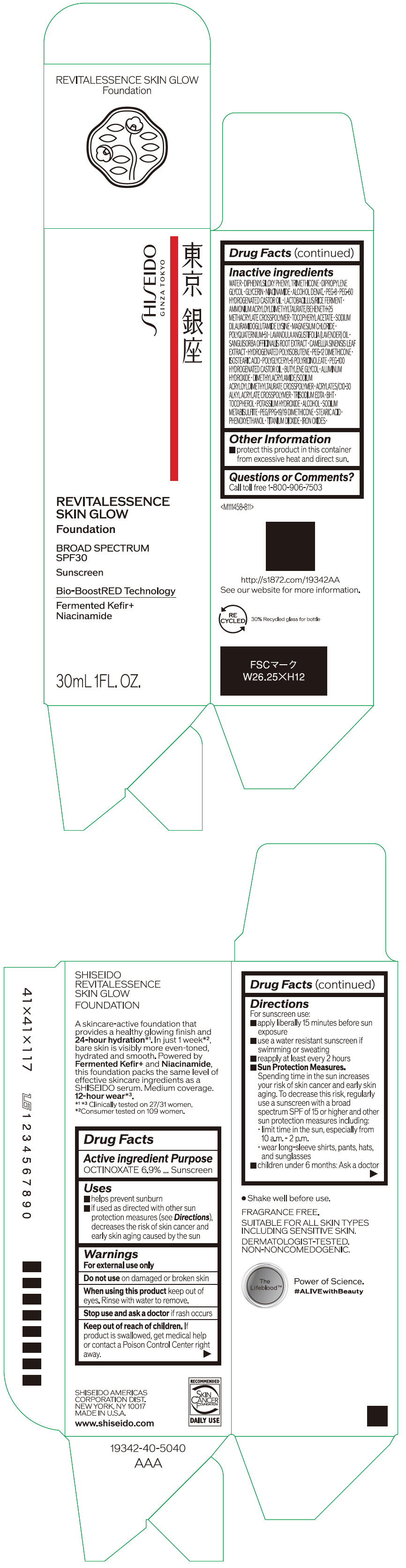
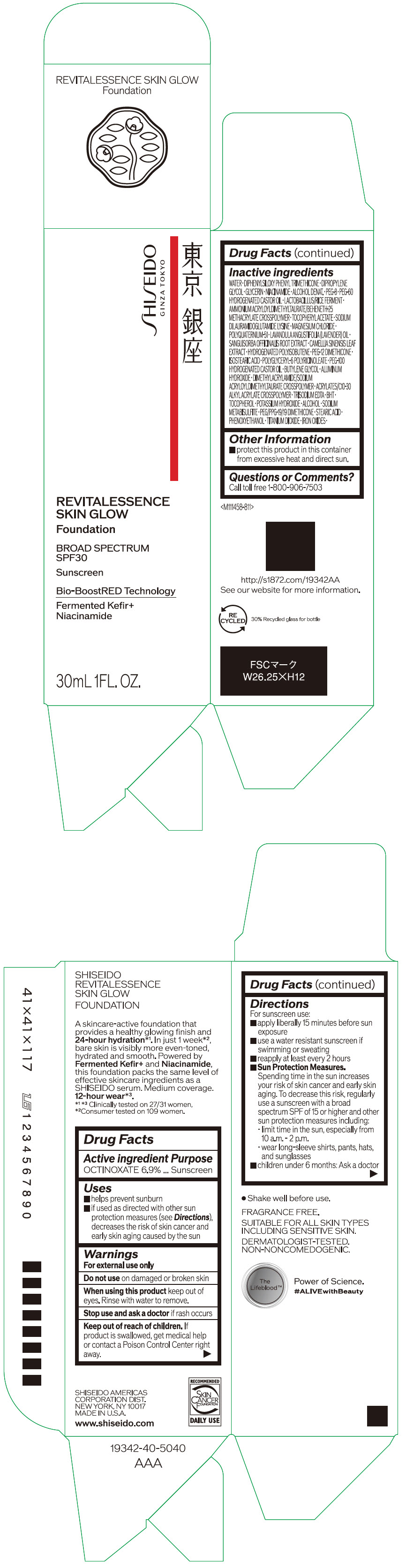
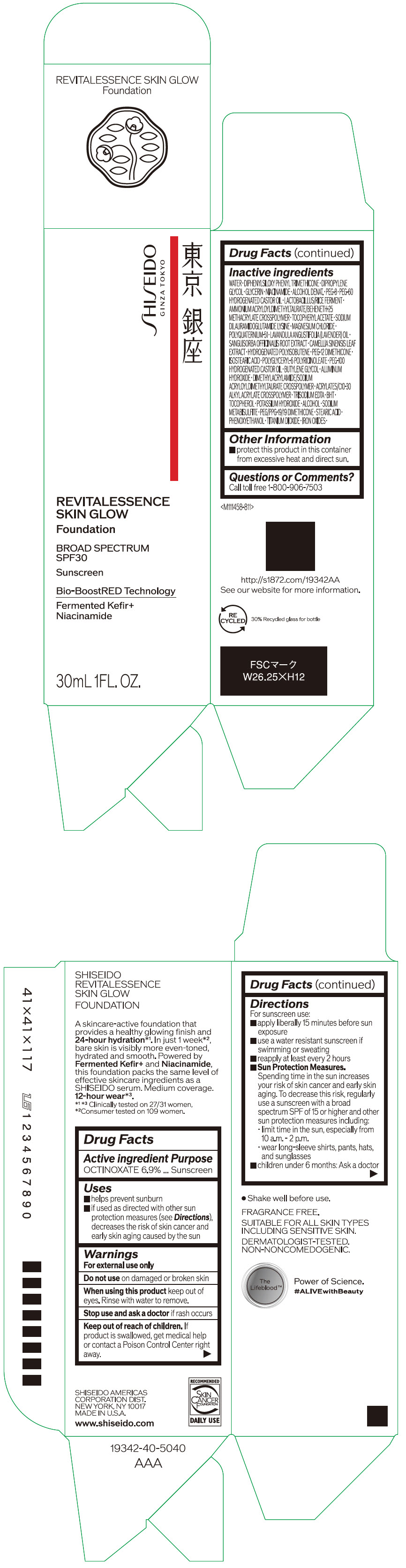
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 110
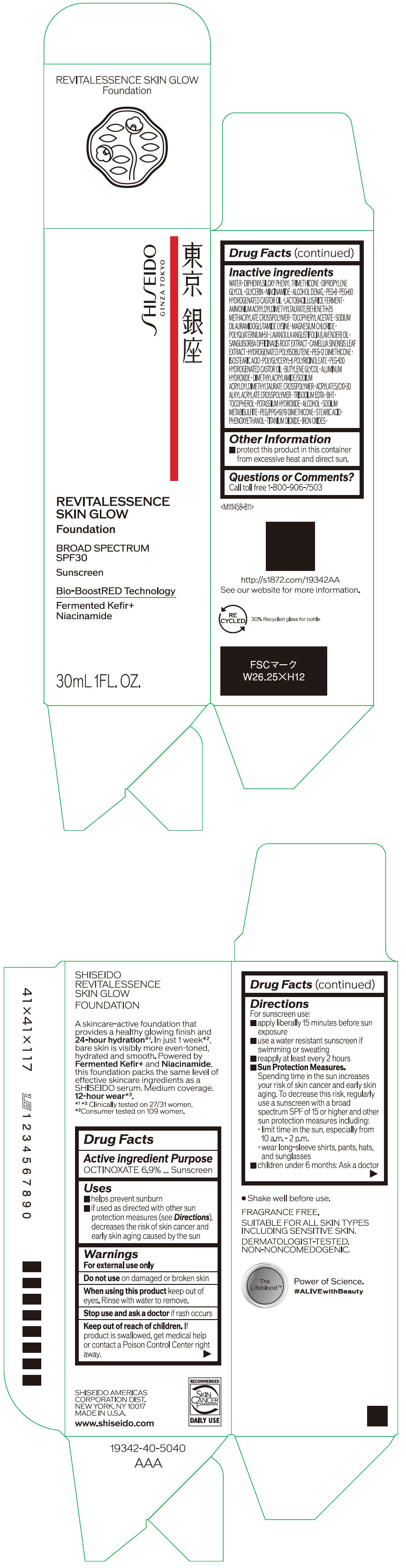
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 120
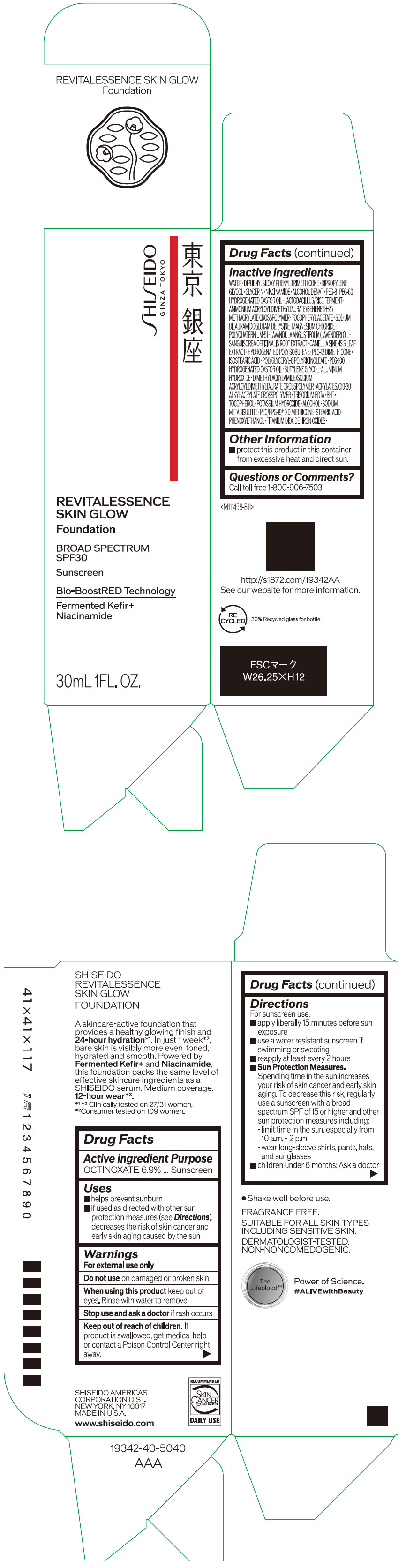
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 130
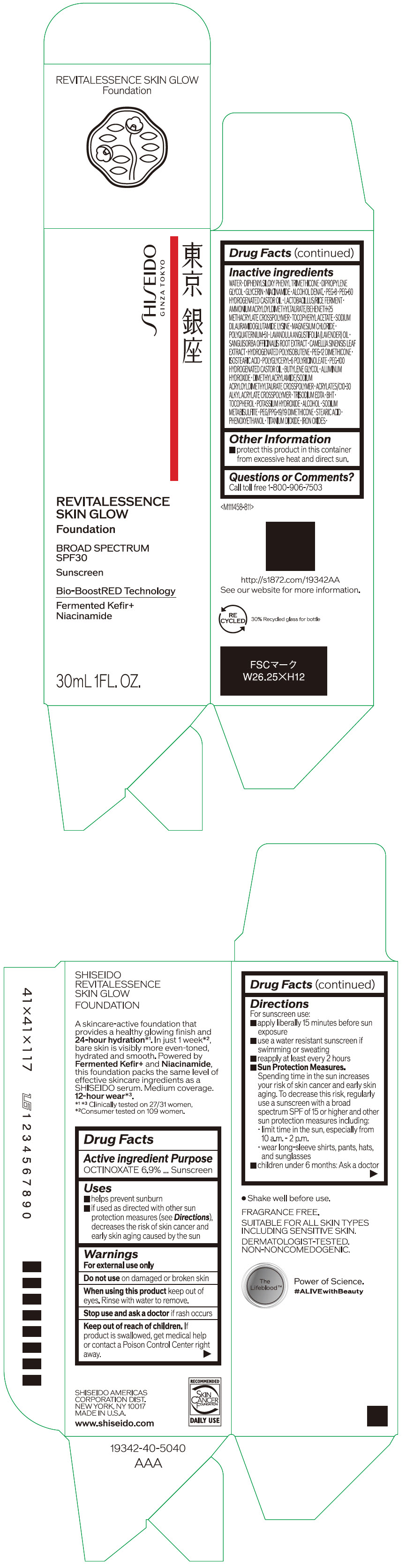
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 140
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 150
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 160
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 210
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 220
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 230
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 240
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 250
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 260
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 310
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 320
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 330
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 340
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 350
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 360
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 410
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 420
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 430
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 440
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 450
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 460
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 510
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 520
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 530
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 540
- PRINCIPAL DISPLAY PANEL - 30 mL Container Carton - 550
-
INGREDIENTS AND APPEARANCE
SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 110
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-859 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-859-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 120
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-860 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-860-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 130
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-861 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-861-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 140
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-862 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-862-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 150
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-863 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-863-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 160
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-864 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-864-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 210
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-865 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-865-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 220
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-866 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-866-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 230
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-867 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-867-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 240
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-868 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-868-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 250
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-869 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-869-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 260
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-870 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-870-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 310
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-871 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-871-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 320
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-872 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-872-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 330
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-873 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-873-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 340
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-874 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-874-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 350
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-875 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-875-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 360
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-876 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-876-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 410
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-877 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-877-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 420
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-878 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-878-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 430
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-879 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-879-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 440
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-880 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-880-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 450
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-881 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-881-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 460
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-882 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-882-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 510
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-883 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-883-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 520
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-884 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-884-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 530
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-885 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-885-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 540
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-886 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-886-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 SHISEIDO REVITALESSENCE SKIN GLOW FOUNDATION 550
octinoxate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-887 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.30805 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) NIACINAMIDE (UNII: 25X51I8RD4) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) PEG-12 DIMETHICONE (300 CST) (UNII: ZEL54N6W95) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) AMMONIUM ACRYLOYLDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER (52000 MPA.S) (UNII: LZ291VH90H) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) EDETATE TRISODIUM (UNII: 420IP921MB) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM METABISULFITE (UNII: 4VON5FNS3C) LAVENDER OIL (UNII: ZBP1YXW0H8) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SANGUISORBA OFFICINALIS ROOT (UNII: 4NYV2HT01X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEG/PPG-19/19 DIMETHICONE (UNII: EHH90CO7TL) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-887-40 1 in 1 CARTON 08/01/2023 1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 08/01/2023 Labeler - SHISEIDO AMERICAS CORPORATION (193691821)