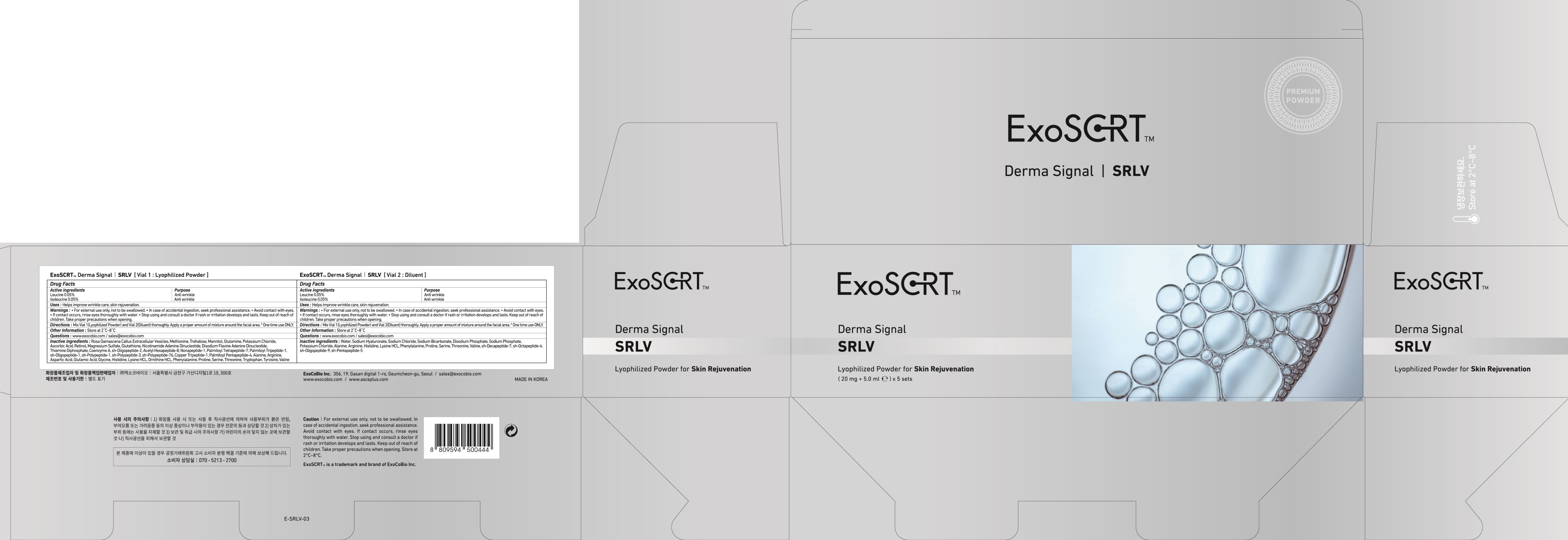
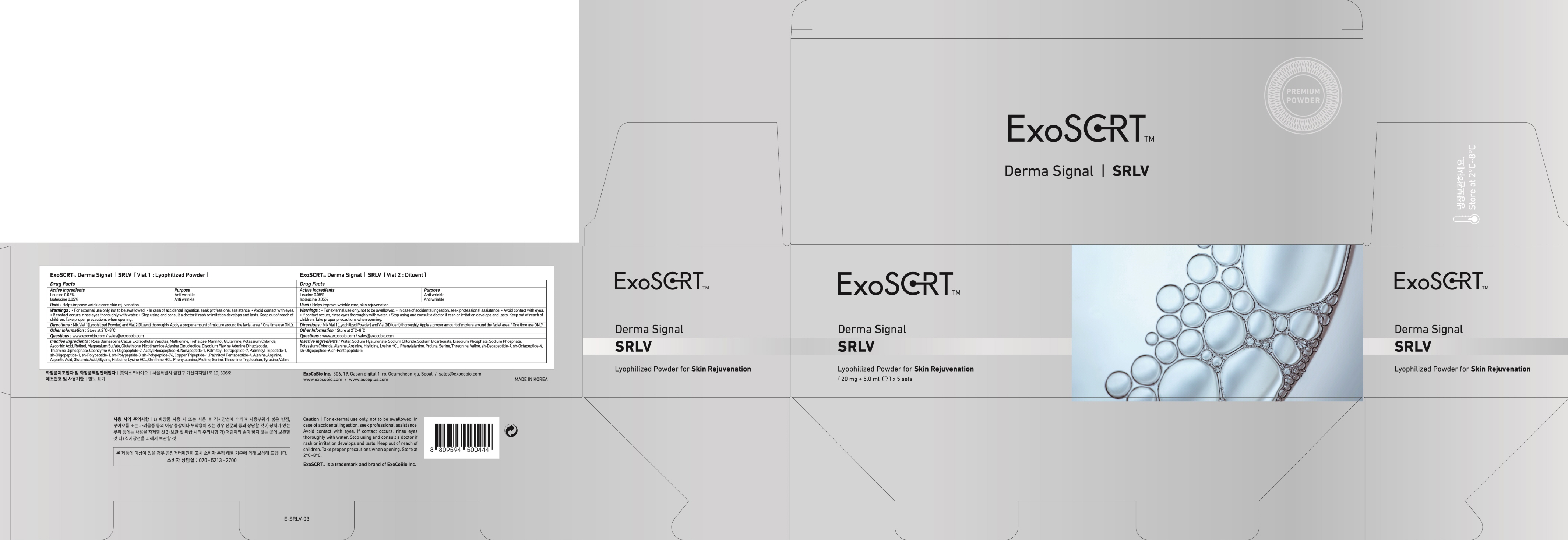
Label: EXOSCRT DERMA SIGNAL SRLV VIAL 1. LYOPHILIZED- leucine, isoleucine powder
- NDC Code(s): 72951-140-01, 72951-140-02
- Packager: Exocobio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENTS
Rosa Damascena Callus Extracellular Vesicles, Methionine, Trehalose, Mannitol, Glutamine, Potassium Chloride, Ascorbic Acid, Retinol, Magnesium Sulfate, Glutathione, Nicotinamide Adenine Dinucleotide, Disodium Flavine, Adenine Dinucleotide, Thiamine Diphosphate, Coenzyme A, sh-Oligopeptide-2, Acetyl Hexapeptide-8, Nonapeptide-1, Palmitoyl Tetrapeptide-7, Palmitoyl Tripeptide-1, sh-Oligopeptide-1, sh-Polypeptide-1, sh-Polypeptide-3, sh-Polypeptide-76, Copper Tripeptide-1, Palmitoyl Pentapeptide-4, Alanine, Arginine, Aspartic Acid, Glutamic Acid, Glycine, Histidine, Lysine HCL, Ornithine HCL, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Tyrosine, Valine
- PURPOSE
-
WARNINGS
For external use only, not to be swallowed.
In case of accidental ingestion, seek professional assistance.
Avoid contact with eyes.
If contact occurs, rinse eyes thoroughly with water.
Stop using and consult a doctor if rash or irritation develops and lasts. Keep out of reach of children. Take proper precautions when opening. - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Other Information
- Questions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXOSCRT DERMA SIGNAL SRLV VIAL 1. LYOPHILIZED
leucine, isoleucine powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72951-140 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Leucine (UNII: GMW67QNF9C) (LEUCINE - UNII:GMW67QNF9C) Leucine 0.01 mg in 20 mg Isoleucine (UNII: 04Y7590D77) (ISOLEUCINE - UNII:04Y7590D77) Isoleucine 0.01 mg in 20 mg Inactive Ingredients Ingredient Name Strength Methionine (UNII: AE28F7PNPL) Trehalose (UNII: B8WCK70T7I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72951-140-02 5 in 1 CARTON 10/01/2021 1 NDC:72951-140-01 20 mg in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2021 Labeler - Exocobio Inc. (694828578) Registrant - Exocobio Inc. (694828578) Establishment Name Address ID/FEI Business Operations Exocobio Inc. 694828578 manufacture(72951-140)