Label: AXUMIN- fluciclovine f-18 injection, solution
- NDC Code(s): 69932-001-30, 69932-001-50
- Packager: Blue Earth Diagnostics
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN.
AXUMIN (fluciclovine F 18) injection, for intravenous use
Initial U.S. Approval: 2016INDICATIONS AND USAGE
Axumin is a radioactive diagnostic agent indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment ( 1).
DOSAGE AND ADMINISTRATION
- Use appropriate radiation safety handling measures ( 2.1).
- Aseptically withdraw Axumin from its container and administer 370 MBq (10 mCi) as a bolus intravenous injection. ( 2.2).
- Initiate imaging 3 minutes to 5 minutes after administration. Scanning should start from mid-thigh and proceed to base of skull, with a total scan time of approximately 20 minutes to 30 minutes ( 2.4).
- The (radiation absorbed) effective dose associated with 370 MBq (10 mCi) of injected activity of Axumin is approximately 8 mSv (0.8 rem) in an adult ( 2.6).
DOSAGE FORMS AND STRENGTHS
Injection: clear, colorless solution in a 30 mL or 50 mL multiple-dose vial containing 335 MBq/mL to 8,200 MBq/mL (9 mCi/mL to 221 mCi/mL) fluciclovine F 18 at calibration time and date ( 3).
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most commonly reported adverse reactions are injection site pain, erythema, and dysgeusia ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Blue Earth Diagnostics, Ltd at 1-855-AXUMIN1 (1-855-298-6461) or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
2.2 Recommended Dose and Administration Instructions
2.3 Patient Preparation Prior to PET Imaging
2.4 Image Acquisition Guidelines
2.5 Image Display and Interpretation
2.6 Radiation Dosimetry
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Image Misinterpretation
5.2 Hypersensitivity Reactions
5.3 Radiation Risks
6 ADVERSE REACTIONS
Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Pediatric Use
8.4 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
11.3 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
Axumin is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration [see Warnings and Precautions ( 5.3) ]. Use waterproof gloves and effective shielding, including syringe shields, when handling and administering Axumin.
2.2 Recommended Dose and Administration Instructions
The recommended dose is 370 MBq (10 mCi) administered as an intravenous bolus injection.
- Inspect Axumin visually for particulate matter and discoloration before administration. Do not use the drug if the solution contains particulate matter or is discolored.
- Use aseptic technique and radiation shielding when withdrawing and administering Axumin.
- Calculate the necessary volume to administer based on calibration time and date, using a suitably calibrated instrument. The recommended maximum volume of injection of undiluted Axumin is 5mL.
- Axumin may be diluted with 0.9% Sodium Chloride Injection, USP.
- After the Axumin injection, administer an intravenous flush of sterile 0.9% Sodium Chloride Injection, USP to ensure full delivery of the dose.
- Dispose of any unused drug in a safe manner in compliance with applicable regulations.
2.3 Patient Preparation Prior to PET Imaging
- Advise the patient to avoid any significant exercise for at least one day prior to PET imaging.
- Advise patients not to eat or drink for at least 4 hours (other than sips of water for taking medications) prior to administration of Axumin.
- Advise patients to void approximately 30 minutes to 60 minutes prior to administration of Axumin and then refrain from voiding until after the scan has been completed
2.4 Image Acquisition Guidelines
Position the patient supine with arms above the head. Begin PET scanning 3 minutes to 5 minutes after completion of the Axumin injection. It is recommended that image acquisition should start from mid-thigh and proceed to the base of the skull. Typical total scan time is between 20 minutes to 30 minutes.
2.5 Image Display and Interpretation
Localization of prostate cancer recurrence in sites typical for prostate cancer recurrence is based on fluciclovine F 18 uptake in comparison with tissue background. For small lesions (less than 1cm in diameter) focal uptake greater than blood pool should be considered suspicious for prostate cancer recurrence. For larger lesions, uptake equal to or greater than bone marrow is considered suspicious for prostate cancer recurrence.
2.6 Radiation Dosimetry
The radiation absorbed doses estimated for adult patients following intravenous injection of Axumin are shown in Table 1. Values were calculated from human biodistribution data using OLINDA/EXM (Organ Level Internal Dose Assessment/Exponential Modeling) software.
The (radiation absorbed) effective dose resulting from the administration of the recommended activity of 370 MBq of Axumin is 8 mSv. For an administered activity of 370 MBq (10 mCi), the highest-magnitude radiation doses are delivered to the pancreas, cardiac wall, and uterine wall: 38 mGy, 19 mGy, and 17 mGy, respectively. If a CT scan is simultaneously performed as part of the PET procedure, exposure to ionizing radiation will increase in an amount dependent on the settings used in the CT acquisition.
Table 1: Estimated Radiation Absorbed Doses in Various Organs/Tissues in Adults who Received Axumin Organ/Tissue Mean Absorbed Dose per Unit Administered Activity (microGy/MBq) Adrenal glands 16 Brain 9 Breasts 14 Gallbladder wall 17 Lower large intestine wall 12 Small intestine wall 13 Stomach wall 14 Upper large intestine wall 13 Heart wall 52 Kidneys 14 Liver 33 Lungs 34 Muscle 11 Ovaries 13 Pancreas 102 Red bone marrow 25 Osteogenic cells 23 Skin 8 Spleen 24 Testes 17 Thymus gland 12 Thyroid 10 Urinary bladder wall 25 Uterus 45 Total body 13 Effective dose 22 (microSv/MBq) - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Image Misinterpretation
Image interpretation errors can occur with Axumin PET imaging. A negative image does not rule out the presence of recurrent prostate cancer and a positive image does not confirm the presence of recurrent prostate cancer. The performance of Axumin seems to be affected by PSA levels [See Clinical Studies ( 14)] . Fluciclovine F 18 uptake is not specific for prostate cancer and may occur with other types of cancer and benign prostatic hypertrophy in primary prostate cancer. Clinical correlation, which may include histopathological evaluation of the suspected recurrence site, is recommended.
5.2 Hypersensitivity Reactions
Hypersensitivity reactions including anaphylaxis may occur in patients who receive Axumin. Emergency resuscitation equipment and personnel should be immediately available.
5.3 Radiation Risks
Axumin use contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Ensure safe handling to minimize radiation exposure to the patient and health care providers [see Dosage and Administration ( 2.1)] .
-
6 ADVERSE REACTIONS
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The clinical trial database for Axumin includes data from 877 subjects including 797 males diagnosed with prostate cancer. Most patients received a single administration of Axumin, a small number of subjects (n = 50) received up to five administrations of the drug. The mean administered activity was 370 MBq (range, 163 MBq to 485 MBq).
Adverse reactions were reported in ≤1% of subjects during clinical studies with Axumin. The most common adverse reactions were injection site pain, injection site erythema and dysgeusia.
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
-
11 DESCRIPTION
11.1 Chemical Characteristics
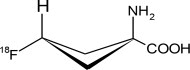
Axumin contains the fluorine 18 (F 18) labeled synthetic amino acid analog fluciclovine. Fluciclovine F 18 is a radioactive diagnostic agent used with PET imaging. Chemically, fluciclovine F 18 is (1r, 3r)-1-amino-3[ 18F]fluorocyclobutane-1-carboxylic acid. The molecular weight is 132.1 and the structural formula is:
Axumin is a sterile, non-pyrogenic, clear, colorless, hyperosmolal (approximately 500 mOsm/kg to 540 mOsm/kg) injection for intravenous use. Each milliliter contains up to 2 micrograms of fluciclovine, 335 MBq to 8,200 MBq (9 mCi to 221 mCi) fluciclovine F 18 at calibration time and date, and 20 mg trisodium citrate in water for injection. The solution also contains hydrochloric acid, sodium hydroxide and has a pH between 4 and 6.
11.2 Physical Characteristics
Fluorine 18 (F 18) is a cyclotron produced radionuclide that decays by positron emission (ß+ decay, 96.7%) and orbital electron capture (3.3%) to stable oxygen 18 with a physical half-life of 109.7 minutes. The positron can undergo annihilation with an electron to produce two gamma rays; the energy of each gamma ray is 511 keV ( Table 2).
Table 2: Principal Radiation Produced from Decay of Fluorine 18 Radiation Energy (keV) Abundance (%) Positron 249.8 96.7 Gamma 511.0 193.5 11.3 External Radiation
The point source air-kerma coefficient for F 18 is 3.75 x 10 -17Gy m 2/(Bq s). The first half-value thickness of lead (Pb) for F 18 gamma rays is approximately 6 mm. The relative reduction of radiation emitted by F 18 that results from various thicknesses of lead shielding is shown in Table 3. The use of 8 cm of Pb will decrease the radiation transmission (i.e., exposure) by a factor of about 10,000.
Table 3: Radiation Attenuation of 511 keV Gamma Rays by Lead Shielding Shield Thickness cm of Lead (Pb) Coefficient of Attenuation 0.6 0.5 2 0.1 4 0.01 6 0.001 8 0.0001 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
Fluciclovine F 18 is a synthetic amino acid transported across mammalian cell membranes by amino acid transporters, such as LAT-1 and ASCT2, which are upregulated in prostate cancer cells. Fluciclovine F 18 is taken up to a greater extent in prostate cancer cells compared with surrounding normal tissues.
12.2 Pharmacodynamics
Following intravenous administration, the tumor-to-normal tissue contrast is highest between 4 and 10 minutes after injection, with a 61% reduction in mean tumor uptake at 90 minutes after injection.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No long term studies in animals have been performed to evaluate the carcinogenic potential of fluciclovine.
Mutagenesis
Fluciclovine was not mutagenic in vitroin reverse mutation assay in bacterial cells and in chromosome aberration test in cultured mammalian cells, and was negative in an in vivoclastogenicity assay in rats after intravenous injection of doses up to 43 mcg/kg. However, fluciclovine F 18 has the potential to be mutagenic because of the F 18 radioisotope.
-
14 CLINICAL STUDIES
The safety and efficacy of Axumin were evaluated in two studies (Study 1 and Study 2) in men with suspected recurrence of prostate cancer based on rising PSA levels following radical prostatectomy and/or radiotherapy.
Study 1 evaluated 105 Axumin scans in comparison to histopathology obtained by biopsy of the prostate bed and biopsies of lesions suspicious by imaging. PET/CT imaging generally included the abdomen and pelvic regions. The Axumin images were originally read by on-site readers. The images were subsequently read by three blinded independent readers. Table 4shows the performance of Axumin in the detection of recurrence in each patient scan and, specifically, within the prostatic bed and extra-prostatic regions, respectively. The results of the independent read were generally consistent with one another and confirmed the results of the on-site reads.
Table 4: Performance of Axumin in Patients with Biochemically Suspected Recurrent Prostate Cancer, at the Patient Level and at the Prostate Bed and Extraprostatic Region Levels N = number of patient scans evaluated
Reader 1 Reader 2 Reader 3 Patient N = 104 N = 105 N = 99 True Positive 75 72 63 False Positive 24 23 13 True Negative 5 7 15 False Negative 0 3 8 Prostate Bed N = 98 N = 97 N = 96 True Positive 58 56 47 False Positive 29 26 15 True Negative 10 12 24 False Negative 1 3 10 Extraprostatic N = 28 N = 28 N = 25 True Positive 25 26 22 False Positive 2 2 2 True Negative 0 0 0 False Negative 1 0 1 The detection rate of Axumin seems to be affected by PSA levels [see Warnings and Precautions ( 5.1)] . In general, patients with negative scans had lower PSA values than those with positive scans. The detection rate (number with positive scans/total scanned) for patients with a PSA value of less than or equal to 1.78 ng/mL (1st PSA quartile) was 15/25, of which 11 were histologically confirmed as positive. In the remaining three PSA quartiles, the detection rate was 71/74, of which 58 were histologically confirmed. Among the 25 patients in the first PSA quartile, there were 4 false positive scans and 1 false negative scan. For the 74 patients with PSA levels greater than1.78 ng/mL, there were 13 false positive scans and no false negative scans.
Study 2 evaluated the concordance between 96 Axumin and C11 choline scans in patients with median PSA value of 1.44 ng/mL (interquartile range = 0.78 ng/mL to 2.8 ng/mL). The C 11 choline scans were read by on-site readers. The Axumin scans were read by the same three blinded independent readers used for Study 1. The agreement values between the Axumin and C11 choline reads were 61%, 67% and 77%, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Axumin is supplied as a clear, colorless injection in a 30 mL or 50 mL multiple-dose glass vial containing approximately 26 mL solution of 335 MBq/mL to 8,200 MBq/mL (9 mCi/mL to 221 mCi/mL) fluciclovine F 18 at calibration time and date.
30 mL sterile multiple-dose vial: NDC 69932-001-30
50 mL sterile multiple-dose vial: NDC 69932-001-50
16.2 Storage and Handling
Store Axumin at controlled room temperature (USP) 20°C to 25°C (68°F to 77°F). Axumin does not contain a preservative. Store Axumin within the original container in radiation shielding. Do not use Axumin more than 10 hours after end of synthesis and dispose of in accordance with institutional guidelines.
This preparation is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
-
17 PATIENT COUNSELING INFORMATION
- Instruct patients to avoid significant exercise for at least a day before the PET scan.
- Instruct patients not to eat or drink for at least 4 hours before the PET scan (other than sips of water for taking medications).
- Instruct patients to try to urinate approximately 30 minutes to 60 minutes prior to planned start time of the PET scan and to avoid further urination until after the scan has been completed.
Marketed by Blue Earth Diagnostics Ltd. Oxford, UK OX4 4GA
Axumin ®is a registered trademark of Blue Earth Diagnostics Ltd.
© 2022 Blue Earth Diagnostics Ltd – all rights reserved. -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 30 mL Multiple-Dose Vial Label
Sterile
Axumin™
Non-pyrogenic
335 MBq/mL to 8,200 MBq/mL (9 mCi/mL to
221 mCi/mL) at End of Synthesis (EOS)Diagnostic - For Intravenous Use Only
Expires 10 hours after EOS
Batch #: ________________________________
EOS Date: __________ EOS Time: __________
Activity @ EOS: ___________________ mCi
Concentration: ___________________ mCi/mL
Volume: ___________________ mL
Exp. Date: __________ Exp. Time: __________
-
INGREDIENTS AND APPEARANCE
AXUMIN
fluciclovine f-18 injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69932-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUCICLOVINE F-18 (UNII: 38R1Q0L1ZE) (FLUCICLOVINE F-18 - UNII:38R1Q0L1ZE) FLUCICLOVINE F-18 221 mCi in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69932-001-30 30 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 05/27/2016 2 NDC:69932-001-50 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 05/27/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208054 05/27/2016 Labeler - Blue Earth Diagnostics (219742530) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Philadelphia) 004201823 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations The University of Utah DBA Cyclotron Radiochemistry Lab, Huntsman Cancer Institute 018432646 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc.(Seattle) 026659644 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Denver) 078575260 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Jacksonville) 111110727 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Portland) 123562576 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (San Antonio) 125764907 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Columbia) 128523862 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (New Orleans) 134146484 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations GE Healthcare AS 515048908 api manufacture(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Phoenix) 603833208 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations P.E.T.NET Houston, LLC 621380547 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Tampa) 788930480 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Hackensack) 796129646 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Tulsa) 798884966 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Dallas) 799246256 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Nashville) 840517945 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Atlanta) 961592982 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Cincinnati) 961593220 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Louisville) 961593337 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Winston-Salem) 961597072 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Cleveland) 961597213 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Albany) 018537881 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Sacramento) 078575242 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Little Rock) 154173350 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Boston) 961597122 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Loma Linda) 079262099 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (San Francisco) 080547824 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Kansas City) 102326340 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Spokane) 798775214 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Detroit) 961593386 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Albuquerque) 961682551 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Minneapolis) 965557486 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions, Inc. (Charlottesville) 968897954 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions Inc (South Florida) 117843428 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions Inc (Las Vegas) 961597221 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions Inc (Chicago 2) 118120165 positron emission tomography drug production(69932-001) Establishment Name Address ID/FEI Business Operations PETNET Solutions Inc. (Culver City) 033883716 positron emission tomography drug production(69932-001)