Label: HYOSCYAMINE SULFATE tablet, extended release
- NDC Code(s): 62559-423-01
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 15, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Hyoscyamine Sulfate Extended-Release Tablets contain 0.375 mg hyoscyamine sulfate in a formulation designed for oral b.i.d. dosage.
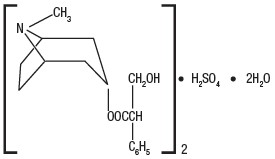
Hyoscyamine sulfate is one of the principal anticholinergic/antispasmodic components of belladonna alkaloids. The empirical formula is (C17H23NO3)2•H2SO4•2H2O and the molecular weight is 712.85. Chemically, it is benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo [3.2.1] oct-3-yl ester, [3(S)-endo]-, sulfate (2:1), dihydrate with the following structure:
Each tablet also contains as inactive ingredients: Colloidal silicon dioxide, hydroxypropyl cellulose, magnesium stearate and microcrystalline cellulose
-
CLINICAL PHARMACOLOGY
Hyoscyamine sulfate inhibits specifically the actions of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that respond to acetylcholine but lack cholinergic innervation. These peripheral cholinergic receptors are present in the autonomic effector cells of the smooth muscle, cardiac muscle, the sinoatrial node, the atrioventricular node and the exocrine glands. At therapeutic doses, it is completely devoid of any action on the autonomic ganglia. Hyoscyamine sulfate inhibits gastrointestinal propulsive motility and decreases gastric acid secretion. Hyoscyamine sulfate also controls excessive pharyngeal, tracheal and bronchial secretions.
Hyoscyamine sulfate is absorbed totally and completely by oral administration. Once absorbed, hyoscyamine sulfate disappears rapidly from the blood and is distributed throughout the entire body. The half-life of hyoscyamine sulfate is 2 to 3 1/2 hours. Hyoscyamine sulfate is partly hydrolyzed to tropic acid and tropine but the majority of the drug is excreted in the urine unchanged within the first 12 hours. Only traces of this drug are found in breast milk. Hyoscyamine sulfate passes the blood brain barrier and the placental barrier.
Hyoscyamine Sulfate Extended-Release Tablets release 0.375 mg hyoscyamine sulfate at a controlled and predictable rate for 12 hours. Tablets may not completely disintegrate and may be excreted by some patients.
-
INDICATIONS AND USAGE
Hyoscyamine Sulfate Extended-Release Tablets are effective as adjunctive therapy in the treatment of peptic ulcer. They can also be used to control gastric secretions, visceral spasm and hypermotility in spastic colitis, spastic bladder, cystitis, pylorospasm and associated abdominal cramps. May be used in functional intestinal disorders to reduce symptoms such as those seen in mild dysenteries, diverticulitis and acute enterocolitis. For use as adjunctive therapy in the treatment of irritable bowel syndrome diverticulitis and acute enterocolitis. For use as adjunctive therapy in the treatment of irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and functional gastrointestinal disorders. Also used as adjunctive therapy in the treatment of neurogenic bladder and neurogenic bowel disturbances (including the splenic flexure syndrome and neurogenic colon). Hyoscyamine Sulfate Extended-Release Tablets are indicated along with morphine or other narcotics in symptomatic relief of biliary and renal colic; as a “drying agent” in the relief of symptoms of acute rhinitis; in the therapy of parkinsonism to reduce rigidity and tremors and to control associated sialorrhea and hyperhidrosis. May be used in the therapy of poisoning by anticholinesterase agents.
-
CONTRAINDICATIONS
Glaucoma; obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis); paralytic ileus, intestinal atony of elderly or debilitated patients; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis.
-
WARNINGS
In the presence of high environmental temperature, heat prostration can occur with drug use (fever and heat stroke due to decreased sweating). Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful. Like other anticholinergic agents, hyoscyamine sulfate may produce drowsiness, dizziness or blurred vision. In this event, the patient should be warned not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery or to perform hazardous work while taking this drug.
Psychosis has been reported in sensitive individuals given anticholinergic drugs including hyoscyamine sulfate. CNS signs and symptoms include confusion, disorientation, short-term memory loss, hallucinations, dysarthria, ataxia, coma, euphoria, anxiety, decreased anxiety, fatigue, insomnia, agitation and mannerisms and inappropriate affect. These CNS signs and symptoms usually resolve within 12 to 48 hours after discontinuation of the drug.
-
PRECAUTIONS
General
Use with caution in patients with: autonomic neuropathy, hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, hypertension and renal disease. Investigate any tachycardia before giving any anticholinergic drugs since they may increase the heart rate. Use with caution in patients with hiatal hernia associated with reflux esophagitis.
Information for Patients
Like other anticholinergic agents, hyoscyamine sulfate may produce drowsiness, dizziness or blurred vision. In this event, the patient should be warned not to engage in activities requiring mental alertness such as operating a motor vehicle or other machinery or to perform hazardous work while taking this drug.
Use of hyoscyamine sulfate may decrease sweating resulting in heat prostration, fever or heat stroke; febrile patients or those who may be exposed to elevated environmental temperatures should use caution. Tablets may not completely disintegrate and may be excreted by some patients.
Drug Interactions
Additive adverse effects resulting from cholinergic blockade may occur when hyoscyamine sulfate is administered concomitantly with other antimuscarinics, amantadine, haloperidol, phenothiazines, monoamine oxidase (MAO) inhibitors, tricyclic antidepressants or some antihistamines.
Antacids may interfere with the absorption of hyoscyamine sulfate.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to determine the carcinogenic, mutagenic or impairment of fertility potential of hyoscyamine sulfate; however, 40 years of marketing experience with hyoscyamine sulfate shows no demonstrable evidence of a problem.
Pregnancy
Animal reproduction studies have not been conducted with hyoscyamine sulfate. It is also not known whether hyoscyamine sulfate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hyoscyamine Sulfate Extended-Release Tablets should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Hyoscyamine sulfate is excreted in human milk. Caution should be exercised when Hyoscyamine Sulfate Extended-Release Tablets are administered to a nursing woman.
Geriatric Use
Reported clinical experience has not identified differences in safety between patients aged 65 and over and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
The following adverse reactions have been reported for hyoscyamine sulfate and for pharmacologically similar drugs with anticholinergic/antispasmodic action. Adverse reactions may include dryness of the mouth; urinary hesitancy and retention; blurred vision; tachycardia; palpitations; mydriasis; cycloplegia; increased ocular tension; loss of taste; headache; nervousness; drowsiness; weakness; fatigue; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; abdominal pain; diarrhea; allergic reactions or drug idiosyncrasies; urticaria and other dermal manifestations; ataxia; speech disturbance; some degree of mental confusion and/or excitement (especially in elderly persons); short-term memory loss; hallucinations; and decreased sweating.
To report SUSPECTED ADVERSE REACTIONS, contact ANI Pharmaceuticals, Inc. at 1-800-308-6755 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
The signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot dry skin, dizziness, dryness of the mouth, difficulty in swallowing and CNS stimulation.
Measures to be taken are immediate lavage of the stomach and injection of physostigmine 0.5 to 2 mg intravenously and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (tepid water sponge baths, hypothermic blanket). Excitement to a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100-200 mL of a 2% solution) by rectal infusion. In the event of progression of the curare-like effect to paralysis of the respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns.
In rats, the LD50 for hyoscyamine is 375 mg/kg. Hyoscyamine sulfate is dialyzable.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Hyoscyamine Sulfate Extended-Release Tablets, 0.375 mg are white, capsule shaped, biconvex tablets debossed with “CL” on one side and “14” on the other.
Bottles of 100 NDC 62559-423-01
Store at controlled room temperature 20° to 25°C (68° to 77°F); excursion permitted to 15° to 30°C (59° to 86°F). Please refer to current USP.
Dispense in tight, light-resistant containers as defined in USP/NF with a child-resistant closure.
KEEP OUT OF REACH OF CHILDREN
Distributed by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

10702 Rev 12/23 - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYOSCYAMINE SULFATE
hyoscyamine sulfate tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62559-423 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYOSCYAMINE SULFATE (UNII: F2R8V82B84) (HYOSCYAMINE - UNII:PX44XO846X) HYOSCYAMINE SULFATE 0.375 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 15mm Flavor Imprint Code CL;14 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62559-423-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 02/15/2024 Labeler - ANI Pharmaceuticals, Inc. (145588013) Registrant - ANI Pharmaceuticals, Inc. (145588013)