Label: PAINFIX RESTORE- menthol patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 72749-000-00 - Packager: PainFix LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
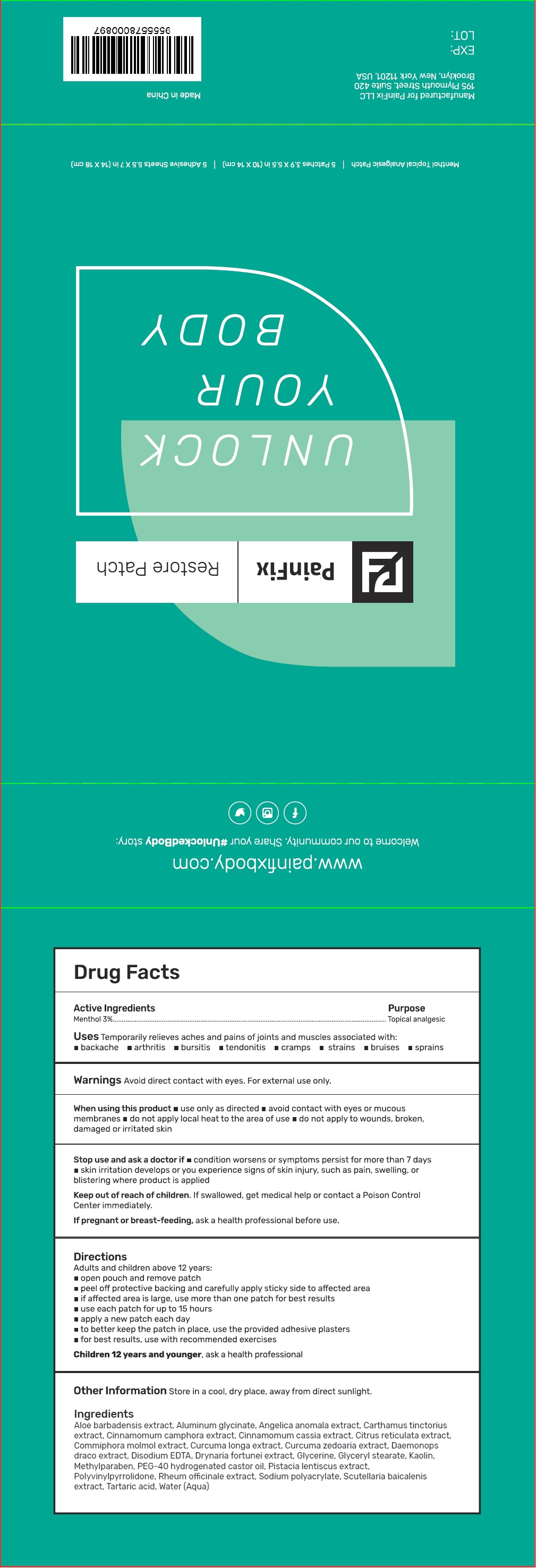
- Drug Facts
- Active Ingredients
- Uses
-
Warnings
Avoid direct contact with eyes. For external use only.
When using this product
• use only as directed • avoid contact with eyes or mucous membranes • do not apply local heat to the area of use • do not apply to wounds, broken, damaged or irritated skin
Stop use and ask a doctor if
• condition worsens or symptoms persist for more than 7 days • skin irritation develops or you experience signs of skin injury, such as pain, swelling, or blistering where product is applied
-
Directions
Adults and children above 12 years:
- open pouch and remove patch
- peel off protective backing and carefully apply sticky side to affected area
- if affected area is large, use more than one patch for best results
- use each patch for up to 15 hours
- apply a new patch each day
- to better keep the patch in place, use the provided adhesive plasters
- for best results, use with recommended exercises
Children 12 years and younger, ask a health professional
- Other Information
-
Ingredients
Aloe barbadensis extract, Aluminum glycinate, Angelica anomala extract, Carthamus tinctorius extract, Cinnamomum camphora extract, Cinnamomum cassia extract, Citrus reticulata extract, Commiphora molmol extract, Curcuma longa extract, Curcuma zedoaria extract, Daemonops draco extract, Disodium EDTA, Drynaria fortunei extract, Glycerine, Glyceryl stearate, Kaolin, Methylparaben, PEG-40 hydrogenated castor oil, Pistacia lentiscus extract, Polyvinylpyrrolidone, Rheum officinale extract, Sodium polyacrylate, Scutellaria baicalenis extract, Tartaric acid, Water (Aqua)
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
PAINFIX RESTORE
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72749-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) TURMERIC (UNII: 856YO1Z64F) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72749-000-00 5 in 1 BOX 02/01/2019 1 1 in 1 PACKET 1 573 mL in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 02/01/2019 Labeler - PainFix LLC (116908667)