Label: STOOL SOFTENER- docusate sodium capsule, liquid filled
- NDC Code(s): 36800-584-25
- Packager: TOP CARE (Topco Associates LLC)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
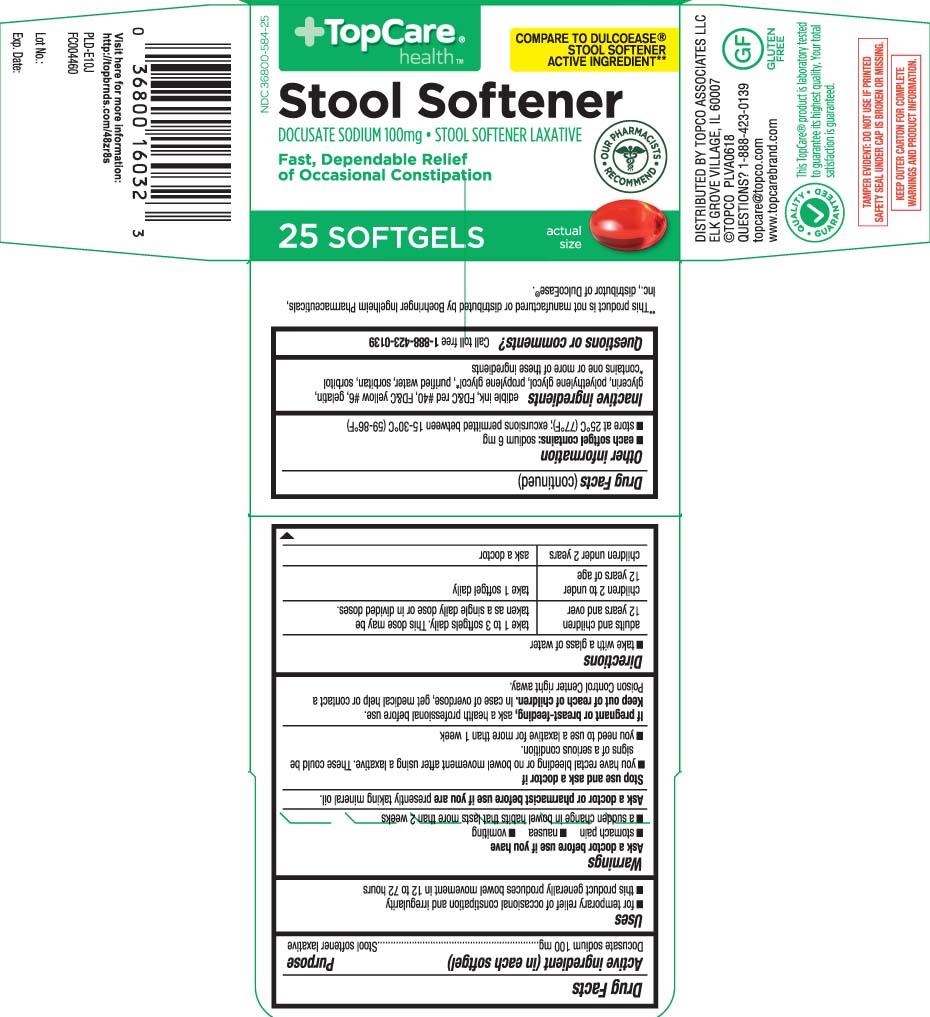
- Active ingredient (in each softgel)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
COMPARE TO DULCOEASE® STOOL SOFTENER ACTIVE INGREDIENT**
Stool Softener
DOCUSATE SODIUM 100 mg • STOOL SOFTENER LAXATIVE
Fast, dependable relief of occasional constipation
SOFTGELS
**This product is not manufactured or distributed by Boehringer Ingelheim Pharmaceuticals, Inc., distributor of DulcoEase®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
DISTRIBUTED BY TOPCO ASSOCIATED LLC
ELK GROVE VILLAGE, IL 60007
©TOPCO PLVA0618
- Product Labeling
-
INGREDIENTS AND APPEARANCE
STOOL SOFTENER
docusate sodium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-584 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color orange (clear) Score no score Shape OVAL Size 12mm Flavor Imprint Code P51;SCU1;D2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-584-25 1 in 1 BOX 07/31/2018 12/31/2024 1 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 07/31/2018 12/31/2024 Labeler - TOP CARE (Topco Associates LLC) (006935977)