Label: COBRAZOL- arnica montana, capsicum annum, crotalus horridus, lachesis muta, naja tripudians gel
- NDC Code(s): 10893-900-03
- Packager: Nature's Innovation, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 14, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
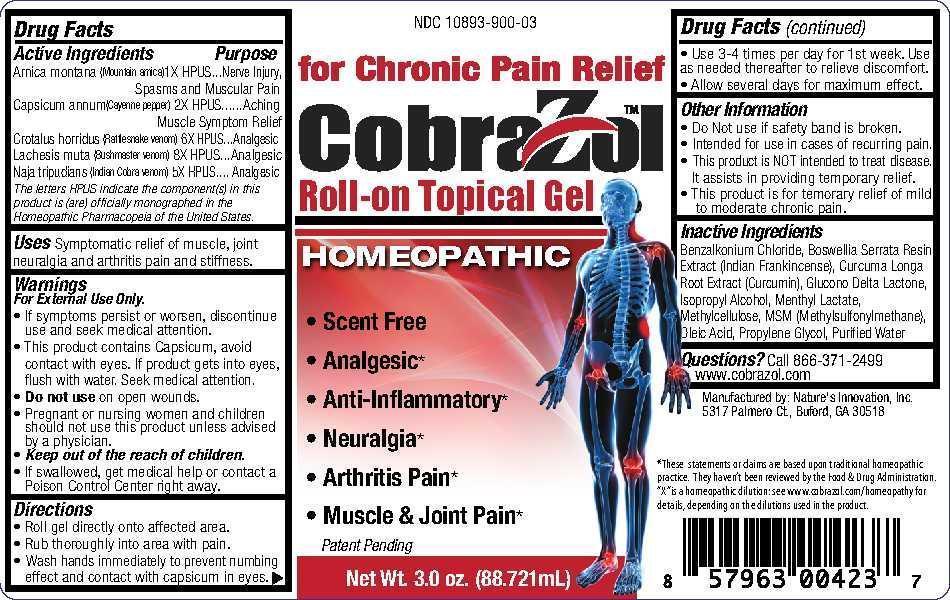
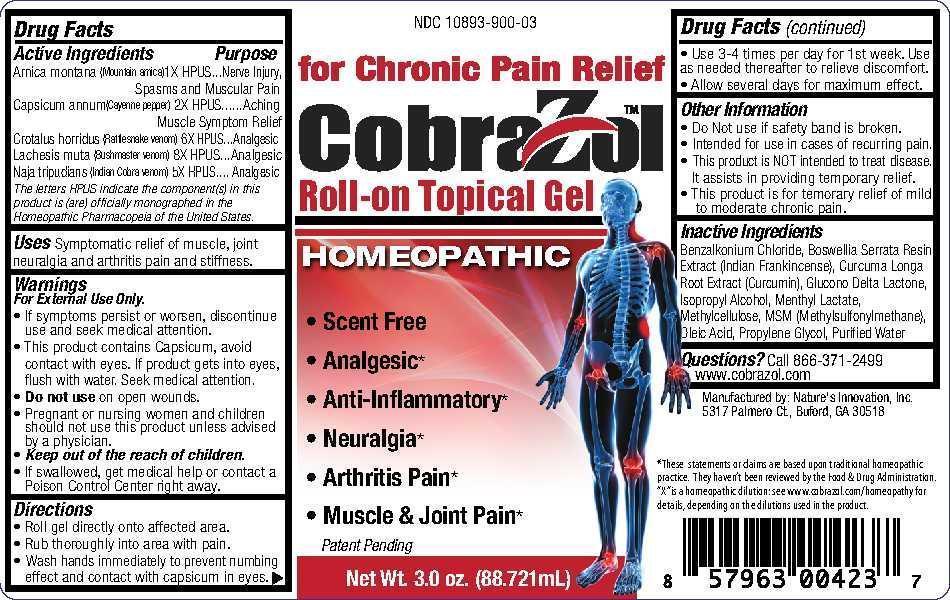
ACTIVE INGREDIENT
Active Ingredients
Arnica montana (Moutain arnica) 1X HPUS
Capsicum annum (Cayenne pepper) 2X HPUS
Crotalus horridus (Rattlesnake venom) 6X HPUS
Lachesis muta (Bushmaster venom) 8X HPUS
Naja tripudians (Indian Cobra venom) 5X HPUS
The letters HPUS indicate the component(s) in this product is (are) officially monographed in the Homeopathic Pharmacopeia of the United States.
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
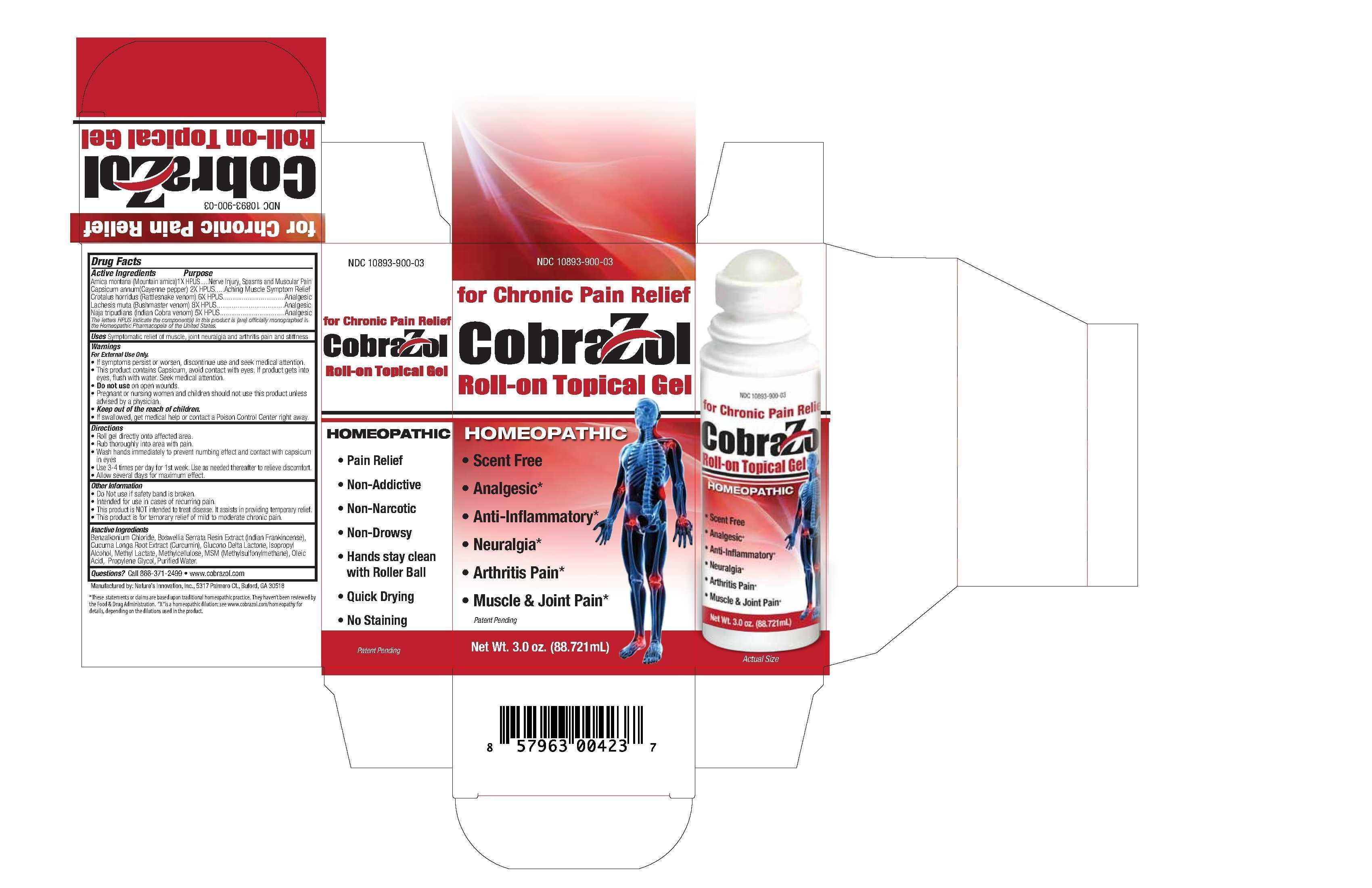
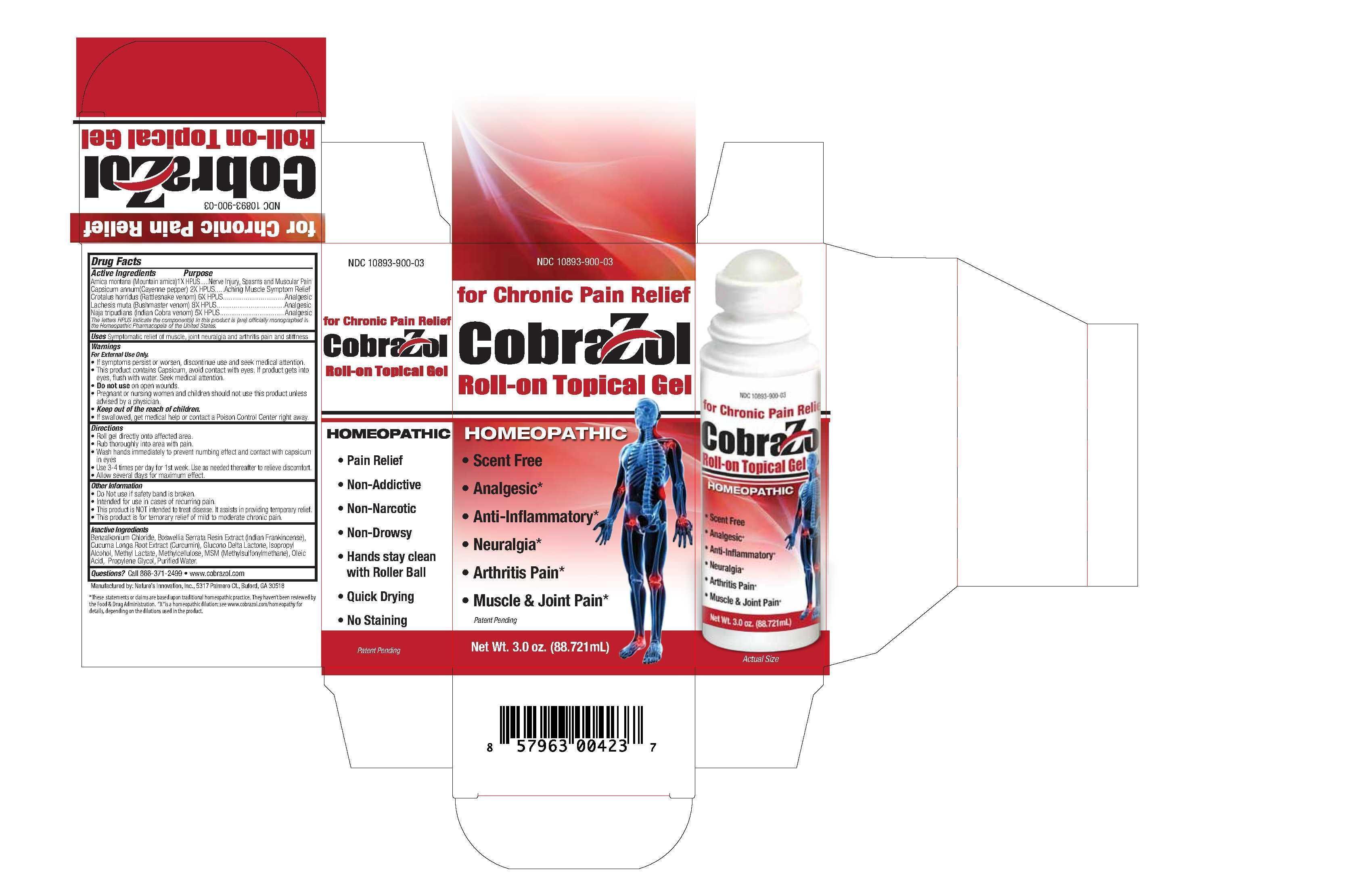
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COBRAZOL
arnica montana, capsicum annum, crotalus horridus, lachesis muta, naja tripudians gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10893-900 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 88.721 mL CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSICUM 2 [hp_X] in 88.721 mL CROTALUS HORRIDUS HORRIDUS VENOM (UNII: YHA2XLJ956) (CROTALUS HORRIDUS HORRIDUS VENOM - UNII:YHA2XLJ956) CROTALUS HORRIDUS HORRIDUS VENOM 6 [hp_X] in 88.721 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 8 [hp_X] in 88.721 mL NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM 5 [hp_X] in 88.721 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) TURMERIC (UNII: 856YO1Z64F) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISOPROPYL ALCOHOL (UNII: ND2M416302) METHYL LACTATE, L- (UNII: 0379G9C44S) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) OLEIC ACID (UNII: 2UMI9U37CP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) METHYLCELLULOSE (1500 CPS) (UNII: P0NTE48364) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10893-900-03 1 in 1 PACKAGE 08/21/2013 1 88.721 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/21/2013 Labeler - Nature's Innovation, Inc. (602969854) Establishment Name Address ID/FEI Business Operations Nature's Innovation, Inc. 602969854 manufacture(10893-900)