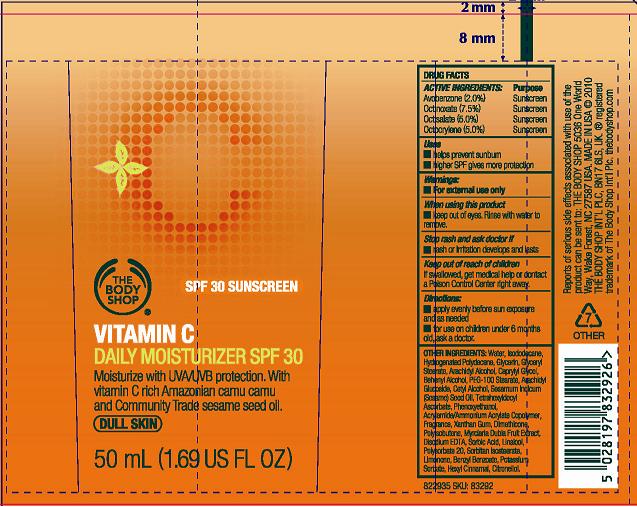
Label: VITAMIN C DAILY MOISTURIZER SPF 30- octinoxate, octisalate, avobenzone, octocrylene lotion
- NDC Code(s): 62282-170-01
- Packager: Northwest Cosmetic Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
WARNINGS
Warnings:
- For external use only
_________________________________________
Do not use
- On damaged or broken skin
_________________________________________
When using this product
- keep out of eyes. Rinse with water to remove.
________________________________________
Stop use and ask doctor if
- rash occurs
________________________________________
Keep out of reach of children
If swallowed, get medical help or contact
a Poison Control Center right away. - DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
OTHER INGREDIENTS: Water, Isododecane,, Hydrogenated Polydecene, Glycerin, Glyceryl Stearate, Arachidyl Alcohol, Caprylyl Glycol, Behenyl Alcohol, PEG 100 Stearate, Arachidyl Glucoside, Cetyl Alcohol, Sesamum Indicum (Sesame) Seed Oil, Tetrahexyldecyl Ascorbate, Phenoxyethanol, Acrylamide/Ammonium Acrylate Copolymer, Fragrance, Xanthan Gum, Dimethicone, Polyisobutene, Myrciaria Dubia Fruit Extract, Disodium EDTA, Sorbic Acid, Linalool, Polysorbate 20, Sorbitan Isostearate, Limonene, Benzyl Benzoate, Potassium Sorbate, Hexyl Cinnamal, Citronellol.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN C DAILY MOISTURIZER SPF 30
octinoxate, octisalate, avobenzone, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62282-170 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) ISODODECANE (UNII: A8289P68Y2) DIMETHICONE (UNII: 92RU3N3Y1O) DOCOSANOL (UNII: 9G1OE216XY) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) POLYOXYL 100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) SESAME OIL (UNII: QX10HYY4QV) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) POLYSORBATE 20 (UNII: 7T1F30V5YH) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62282-170-01 50 mL in 1 TUBE; Type 0: Not a Combination Product 06/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/01/2010 Labeler - Northwest Cosmetic Laboratories (929572014)