Label: PAINZONE- external analgesic cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 70338-641-10, 70338-641-12, 70338-641-30 - Packager: MedZone Products LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 12, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
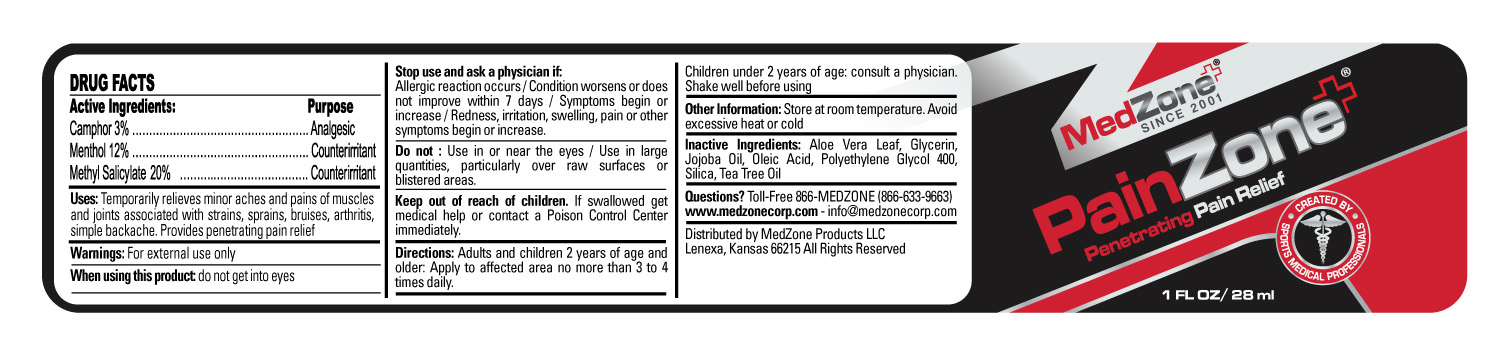
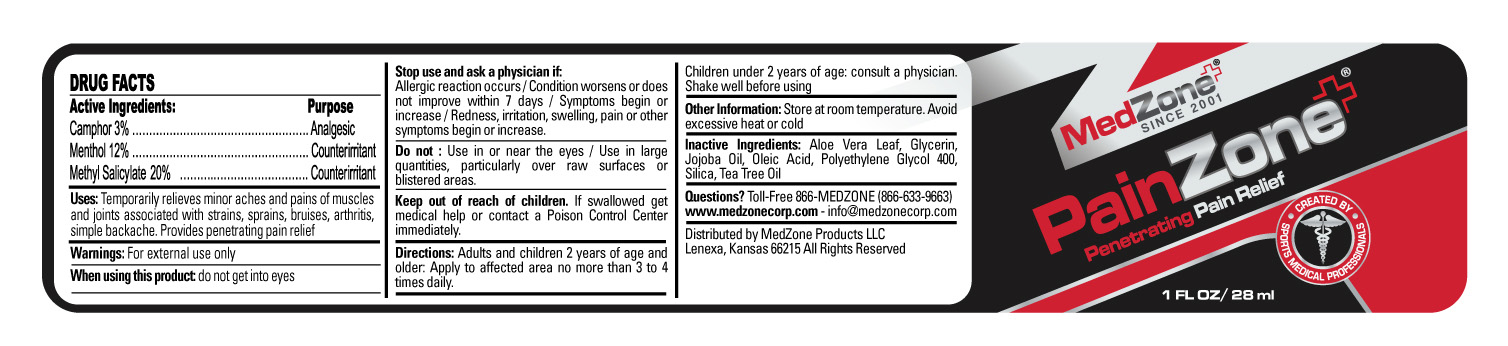
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.

Stop use and ask a physician if
- Allergic reaction occurs
- Condition worsens or does not improve within 7 days
- Symptoms clear up and return within a few days
- Redness, irritation, swelling, pain or other symptoms begin or increase
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAINZONE
external analgesic creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70338-641 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR, (-)- (UNII: 213N3S8275) (CAMPHOR, (-)- - UNII:213N3S8275) CAMPHOR, (-)- 30 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 120 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) JOJOBA OIL (UNII: 724GKU717M) OLEIC ACID (UNII: 2UMI9U37CP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70338-641-12 3.6 g in 1 POUCH; Type 0: Not a Combination Product 07/01/2016 2 NDC:70338-641-30 88 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 09/30/2016 3 NDC:70338-641-10 28 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 09/30/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/01/2016 Labeler - MedZone Products LLC (080083739) Registrant - Richard Hamer Associates LLC (067731889) Establishment Name Address ID/FEI Business Operations Biomed Laboratories LLC 055329696 pack(70338-641) , manufacture(70338-641) Establishment Name Address ID/FEI Business Operations MicroConsult Inc 062183608 analysis(70338-641) Establishment Name Address ID/FEI Business Operations Ceutical Laboratories Inc 789485153 analysis(70338-641)