Label: METHAZOLAMIDE tablet
- NDC Code(s): 51224-024-50, 51224-124-50
- Packager: TAGI Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Methazolamide, a sulfonamide derivative, is a white or faintly yellow, crystalline powder, soluble in dimethyl formamide, slightly soluble in acetone, very slightly soluble in water and alcohol. The chemical name for methazolamide is:
Acetamide, N-[5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazol-2(3H)-ylidene]- and it has the following structural formula:
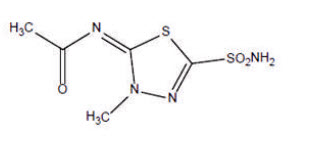
C5H8N4O3S2
236.26Each tablet, for oral administration, contains 25 mg or 50 mg methazolamide. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, magnesium stearate, microcrystalline cellulose, and isopropyl alcohol.
Isopropyl alcohol is removed during manufacturing process
-
CLINICAL PHARMACOLOGY
Methazolamide is a potent inhibitor of carbonic anhydrase.
Methazolamide is well absorbed from the gastrointestinal tract. Peak plasma concentrations are observed 1 to 2 hours after dosing. In a multiple-dose, pharmacokinetic study, administration of methazolamide 25 mg BID, 50 mg BID, and 100 mg BID demonstrated a linear relationship between plasma methazolamide levels and methazolamide dose. Peak plasma concentrations (Cmax) for the 25 mg, 50 mg and 100 mg BID regimens were 2.5 mcg/mL, 5.1 mcg/mL, and 10.7 mcg/mL, respectively. The area under the plasma concentration-time curves (AUC) were 1130 mcg.min/mL, 2571 mcg.min/mL, and 5418 mcg.min/mL for the 25 mg, 50 mg, and 100 mg dosage regimens, respectively.
Methazolamide is distributed throughout the body including the plasma, cerebrospinal fluid, aqueous humor of the eye, red blood cells, bile and extracellular fluid. The mean apparent volume of distribution (V area /F) ranges from 17 L to 23 L. Approximately 55% is bound to plasma proteins. The steady-state methazolamide red blood cell:plasma ratio varies with dose and were found to be 27:1, 16:1, and 10:1 following the administration of methazolamide 25 mg BID, 50 mg BID, and 100 mg BID, respectively.
The mean steady-state plasma elimination half-life for methazolamide is approximately 14 hours. At steady-state, approximately 25% of the dose is recovered unchanged in the urine over the dosing interval. Renal clearance accounts for 20% to 25% of the total clearance of drug. After repeated BID- tid dosing, methazolamide accumulates to steady-state concentrations in 7 days.
Methazolamide's inhibitory action on carbonic anhydrase decreases the secretion of aqueous humor and results in a decrease in intraocular pressure. The onset of the decrease in intraocular pressure generally occurs within 2 to 4 hours, has a peak effect in 6 to 8 hours and a total duration of 10 to 18 hours.
Methazolamide is a sulfonamide derivative; however, it does not have any clinically significant antimicrobial properties. Although methazolamide achieves a high concentration in the cerebrospinal fluid, it is not considered an effective anticonvulsant.
Methazolamide has a weak and transient diuretic effect; therefore, use results in an increase in urinary volume, with excretion of sodium, potassium, and chloride. The drug should not be used as a diuretic.
Inhibition of renal bicarbonate reabsorption produces an alkaline urine. Plasma bicarbonate decreases,and a relative, transient metabolic acidosis may occur due to a disequilibrium in carbon dioxide transport in the red blood cell. Urinary citrate excretion is decreased by approximately 40% after doses of 100 mg every 8 hours. Uric acid output has been shown to decrease 36% in the first 24 hour period.
-
INDICATIONS AND USAGE
Methazolamide Tablets, USP is indicated in the treatment of ocular conditions where lowering intraocular pressure is likely to be of therapeutic benefit, such as chronic open-angle glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where lowering the intraocular pressure is desired before surgery.
-
CONTRAINDICATIONS
Methazolamide therapy is contraindicated in situations in which sodium and/or potassium serum levels are depressed, in cases of marked kidney or liver disease or dysfunction, in adrenal gland failure, and in hyperchloremic acidosis. In patients with cirrhosis, use may precipitate the development of hepatic encephalopathy.
Long-term administration of methazolamide is contraindicated in patients with angle-closure glaucoma, since organic closure of the angle may occur in spite of lowered intraocular pressure.
-
WARNINGS
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Hypersensitivity reactions may recur when a sulfonamide is readministered, irrespective of the route of administration.
If hypersensitivity or other serious reactions occur, the use of this drug should be discontinued.
Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly, as anorexia, tachypnea, lethargy, coma, and death have been reported with concomitant use of high-dose aspirin and carbonic anhydrase inhibitors.
-
PRECAUTIONS
General
Potassium excretion is increased initially upon administration of methazolamide and in patients with cirrhosis or hepatic insufficiency could precipitate a hepatic coma.
In patients with pulmonary obstruction or emphysema, where alveolar ventilation may be impaired, methazolamide should be used with caution because it may precipitate or aggravate acidosis.
Information for Patients
Adverse reactions common to all sulfonamide derivatives may occur: anaphylaxis, fever, rash (including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), crystalluria, renal calculus, bone marrow depression, thrombocytopenic purpura, hemolytic anemia, leukopenia, pancytopenia, and agranulocytosis. Precaution is advised for early detection of such reactions, and the drug should be discontinued and appropriate therapy instituted.
Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly.
Laboratory Tests
To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating methazolamide therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum electrolytes is also recommended.
Drug Interactions
Methazolamide should be used with caution in patients on steroid therapy because of the potential for developing hypokalemia.
Caution is advised for patients receiving high-dose aspirin and methazolamide concomitantly, as anorexia, tachypnea, lethargy, coma and death have been reported with concomitant use of high-dose aspirin and carbonic anhydrase inhibitors (see WARNINGS).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate methazolamide's carcinogenic potential and its effect on fertility have not been conducted. Methazolamide was not mutagenic in the Ames bacterial test.
Pregnancy
Teratogenic effects. Methazolamide has been shown to be teratogenic (skeletal anomalies) in rats when given in doses approximately 40 times the human dose. There are no adequate and well controlled studies in pregnant women.
Methazolamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from methazolamide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
Adverse reactions, occurring most often early in therapy, include paresthesias, particularly a "tingling" feeling in the extremities; hearing dysfunction or tinnitus; fatigue; malaise; loss of appetite; taste alteration; gastrointestinal disturbances such as nausea, vomiting, and diarrhea; polyuria; and occasional instances of drowsiness and confusion.
Metabolic acidosis and electrolyte imbalance may occur.
Transient myopia has been reported. This condition invariably subsides upon diminution or discontinuance of the medication.
Other occasional adverse reactions include urticaria, melena, hematuria, glycosuria, hepatic insufficiency, flaccid paralysis, photosensitivity, convulsions, and, rarely, crystalluria and renal calculi.
Also see PRECAUTIONS: Information for Patients for possible reactions common to sulfonamide derivatives. Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias (see WARNINGS).
-
OVERDOSAGE
No data are available regarding methazolamide overdosage in humans as no cases of acute poisoning with this drug have been reported. Animal data suggest that even a high dose of methazolamide is nontoxic. No specific antidote is known. Treatment should be symptomatic and supportive.
Electrolyte imbalance, development of an acidotic state, and central nervous system effects might be expected to occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
Supportive measures may be required to restore electrolyte and pH balance.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Methazolamide Tablets USP, 25 mg are white to off white, uncoated, round, biconvex tablets debossed with '25' on one side and 'ZEN' on other side and are supplied in bottles of 100, NDC 51224-024-50.
Methazolamide Tablets USP, 50 mg are white to off white, uncoated, round, biconvex tablets debossed with '5 and 0' on either side of the break line one side and 'ZEN' on other side and are supplied in bottles of 100, NDC 51224-124-50.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
METHAZOLAMIDE
methazolamide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51224-024 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methazolamide (UNII: W733B0S9SD) (Methazolamide - UNII:W733B0S9SD) Methazolamide 25 mg Inactive Ingredients Ingredient Name Strength Dibasic Calcium Phosphate Dihydrate (UNII: O7TSZ97GEP) Microcrystalline cellulose (UNII: OP1R32D61U) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Silicon Dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code 25;ZEN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51224-024-50 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215615 01/01/2022 METHAZOLAMIDE
methazolamide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51224-124 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methazolamide (UNII: W733B0S9SD) (Methazolamide - UNII:W733B0S9SD) Methazolamide 50 mg Inactive Ingredients Ingredient Name Strength Dibasic Calcium Phosphate Dihydrate (UNII: O7TSZ97GEP) Microcrystalline cellulose (UNII: OP1R32D61U) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Silicon Dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 7mm Flavor Imprint Code 50;ZEN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51224-124-50 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215615 01/01/2022 Labeler - TAGI Pharma, Inc. (963322560) Establishment Name Address ID/FEI Business Operations Zenara Pharma Private Limited 924839850 MANUFACTURE(51224-024, 51224-124)




