Label: NEOBENZ(R) MICRO BENZOYL PEROXIDE WASH, 7%- benzoyl peroxide, 7% lotion
- NDC Code(s): 67402-027-17
- Packager: SkinMedica Pharmaceuticals, Inc., Carlsbad, CA 92010
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 6, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
I. DESCRIPTION
NeoBenz® Micro 3.5% and 5.5% creams, NeoBenz® Micro SD 5.5% single dose cream and NeoBenz® Micro Wash 7% are topical preparations containing benzoyl peroxide as the active ingredient incorporated into patented porous microspheres (MICROSPONGE®* delivery system) composed of methyl methacrylate/glycol dimethacrylate crosspolymer. This polymeric system has been shown to provide gradual release of active ingredient into the skin1 and absorb natural skin oils2. Ingredients for all cream and SD strengths include: water, glycerin, ethylhexyl palmitate, sorbitol, cetyl alcohol, glyceryl ditaurate, stealryl alcohol, magnesium aluminum silicate, methyl methacrylate/glycol dimethacrylate crosspolymer, silica, citric acid, xanthan gum, methylparaben, sodium citrate, propyIparaben, polyacrylamide, C13-14 isoparaffin, laureth-7, sodium lauryl sulfate. Ingredients for the Wash include citric acid, cocamidopropyl betaine, cocamine oxide, disodium laureth sulfosuccinate, edetate disodium, fragrance, glycerin, hydrogenated castor oil, hypromellose, magnesium aluminum silicate, methyl methacrylate/Glycol dimethacrylate crosspolymer, methylparaben, PEG-150 pentaerythrityl tetrastearate (and) PEG-6 caprylic/capric glycerides, PEG-40 hydrogenated castor oil, poloxamer 182, purified water and xanthan gum.
Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following structure:
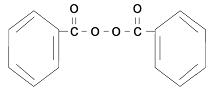
-
II. CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide is believed to be responsible for its usefulness in acne. Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
- III. INDICATIONS AND USAGE
- IV. CONTRAINDICATIONS
- V. WARNINGS
-
VI. PRECAUTIONS
(SEE WARNINGS)
General– For external use only. Avoid contact with eyes and mucous membranes. If severe irritation develops, discontinue use and institute appropriate therapy. -
INFORMATION FOR PATIENTS
Information for Patients: Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. AVOID CONTACT WITH HAIR, FABRICS OR CARPETING AS BENZOYL PEROXIDE WILL CAUSE BLEACHING OR DISCOLORATION. If excessive irritation develops, discontinue use and consult your physician.
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of Fertility: Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
-
PREGNANCY
Pregnancy: Category C - Animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed.
- NURSING MOTHERS
- PEDIATRIC USE
- VII . ADVERSE REACTIONS
-
VIII. OVERDOSAGE
If excessive scaling, erythema or edema occurs, the use of these preparations should be discontinued. To hasten resolution of the adverse effects, cool compresses may be used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not allergenicity.
-
IX. DOSAGE AND ADMINISTRATION
NeoBenz® Micro, NeoBenz® Micro SD and NeoBenz® Micro Wash should be used once or twice daily on the affected areas. Frequency of use should be adjusted to obtain the desired clinical response. If you see medication or white residue on skin after application of NeoBenz® Micro or NeoBenz® Micro SD, you are applying too much. Gentle cleansing of the affected areas with a mild cleanser prior to application of NeoBenz® Micro or NeoBenz® Micro SD may be beneficial. Clinically visible improvement will normally occur by the third week if therapy. Maximum lesion reduction may be expected after approximately eight to twelve weeks for drug use. Continuing use of the drug us normally required to maintain a satisfactory clinical response.
NeoBenz® Micro: If applying to the entire face, apply a pea-sized amount of NeoBenz® Micro to one fingertip and dab onto cheeks, forehead and chin. Spread evenly onto entire face.
NeoBenz® Micro SD: Firmly squeeze applicator until seal between applicator and sponge has broken. Apply medication by rubbing sponge on small circular motions on affected areas. Dispose of each applicator after single use only.
NeoBenz® Micro Wash: Shake well before using. Wet skin areas to be treated; apply NeoBenz® Micro Wash by massaging gently into skin for 10-20 seconds, working into a full lather. Rinse thoroughly and pat dry. -
X. HOW SUPPLIED
NeoBenz® Micro is supplied as follows:
SIZE: 45 gram tube
NDC NUMBER:
(3.5% benzoyl peroxide cream): 67402-020-45
(5.5% benzoyl peroxide cream): 67402-021-45
NeoBenz® Micro SD is supplied as follows:
SIZE: 1 box of thirty 0.5 gram applicators
NDC NUMBER:
(5.5% benzoyl peroxide cream): 67402-021-06
NeoBenz® Micro Wash is supplied as follows:
SIZE: 180 gram tottle
NDC NUMBER:
(benzoyl peroxide 7%): 67402-027-17
- STORAGE AND HANDLING
-
REFERENCES
1. Wester RC, Patel R, Nacht S, Leyden J, Melendres J, Maibach H. Controlled release of benzoyl peroxide from a porous microsphere polymeric system can reduce topical irritancy. J. Am. Acad. Dermatol. 1991;24; 720-726.
2. Meyer R. Rosen (ed.), Delivery System Handbook for Personal care and Cosmetic Products, 332-352 (2005). - SPL UNCLASSIFIED SECTION
- NeoBenz Micro Wash, Benzoyl Peroxide 7%
-
INGREDIENTS AND APPEARANCE
NEOBENZ(R) MICRO BENZOYL PEROXIDE WASH, 7%
benzoyl peroxide, 7% lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67402-027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 70 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67402-027-17 180 g in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved other 12/15/2008 Labeler - SkinMedica Pharmaceuticals, Inc., Carlsbad, CA 92010 (124977005)




