Label: CHILDRENS PAIN RELIEF- acetaminophen tablet, chewable
- NDC Code(s): 69842-160-24
- Packager: CVS Pharmacy,Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
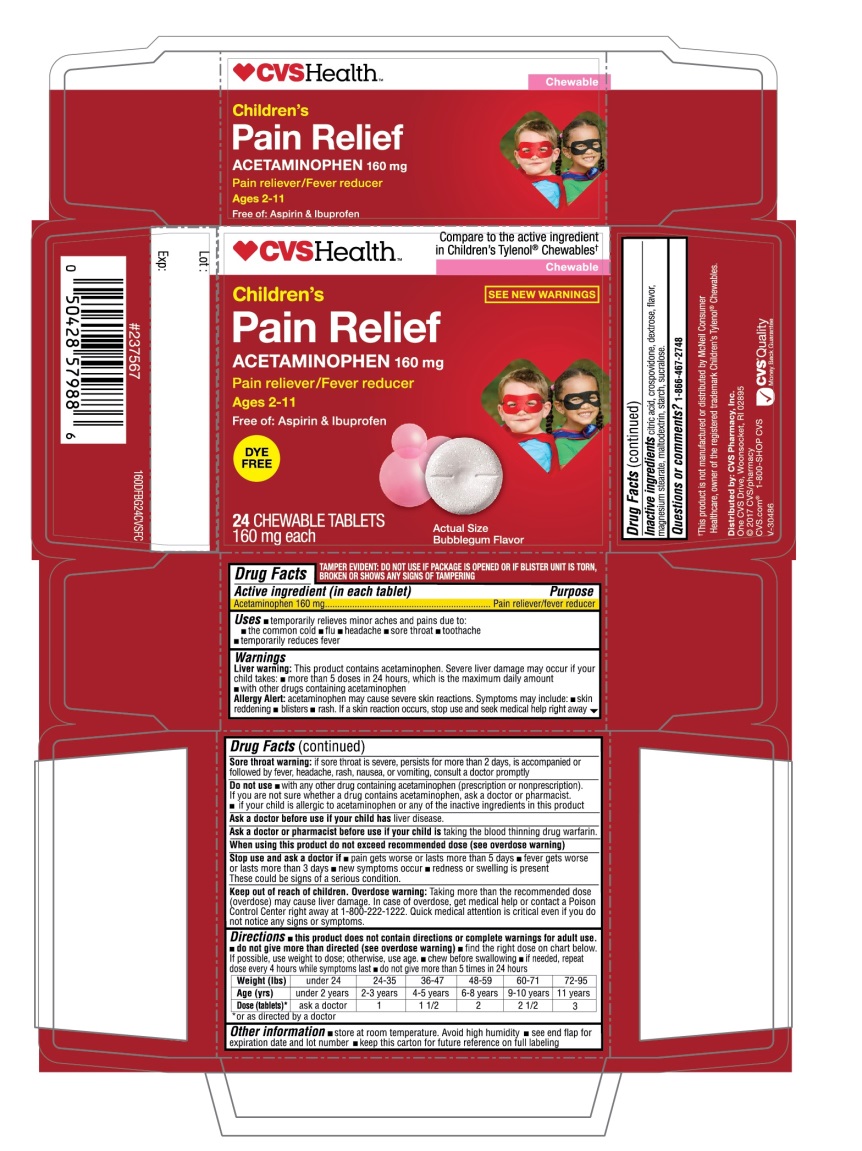
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
- ▪
- more than 5 doses in 24 hours, which is the maximum daily amount
- ▪
- with other drugs containing acetaminophen.
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- ▪
- skin reddening
- ▪
- blisters
- ▪
- rash
If a skin reaction occurs, stop use and seek medical help right away
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly
Do not use
- ▪
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- ▪
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product.
Stop use and ask a doctor if
- ▪
- pain gets worse or lasts more than 5 days
- ▪
- fever gets worse or lasts more than 3 days
- ▪
- new symptoms occur
- ▪
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away at 1-800-222-1222. Quick medical attention is critical even if you do not notice any signs or symptoms.
-
Directions
- ▪
- this product does not contain directions or complete warnings for adult use.
- ▪
- do not give more than directed (see overdose warning)
- ▪
- find the right dose on chart below. If possible, use weight to dose; otherwise use age.
- ▪
- chew before swallowing
- ▪
- if needed, repeat dose every 4 hours while symptoms last
- ▪
- do not give more than 5 times in 24 hours
Weight (lbs)
under 24
24-35
36-47
48-59
60-71
72-95
Age (yrs)
under 2 years
2-3- years
4-5 years
6-8 years
9-10 years
11 years
Dose(tablets)*
ask a doctor
1
1 1/2
2
2 1/2
3
- *or as directed by a doctor
- Other information
- Inactive ingredients
-
Principal Display Panel
NDC 69842-160-24
Compare to the active ingredient in Children’s Tylenol® Chewables†
Children’s
Pain Relief
Acetaminophen 160 mg
Pain reliever/Fever reducer
Ages 2-11
Free of: Aspirin & Ibuprofen
BUBBLEGUM FLAVOR
24 CHEWABLE TABLETS
160 mg each
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
†This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Children’s Tylenol® Chewables.
Distributed by: CVS Pharmacy, Inc,
One CVS Drive, Woonsocket, RI 02895
©2017 CVS/Pharmacy
CVS.com 1-800-SHOP CVS
-
INGREDIENTS AND APPEARANCE
CHILDRENS PAIN RELIEF
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-160 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) STARCH, CORN (UNII: O8232NY3SJ) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 16mm Flavor BUBBLE GUM Imprint Code RP160 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-160-24 4 in 1 CARTON 11/01/2017 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 11/01/2017 Labeler - CVS Pharmacy,Inc. (062312574)