Label: LBEL- octinoxate and oxybenzone lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-011-61, 13537-011-62 - Packager: VENTURA INTERNATIONAL LTD.,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 10, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
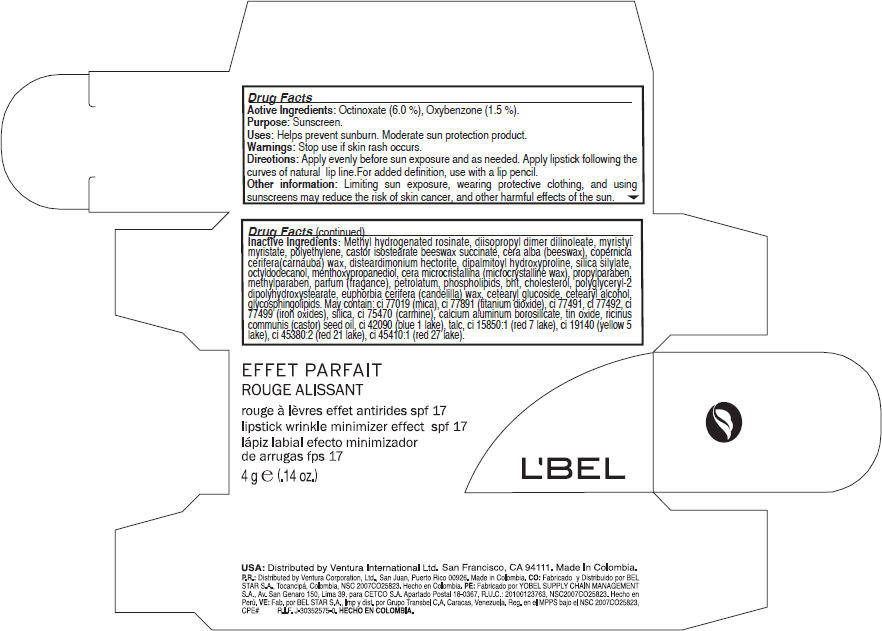
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive Ingredients
Methyl hydrogenated rosinate, diisopropyl dimer dilinoleate, myristyl myristate,myristyl myristate, castor isostearate beeswax succinate, cera alba (beeswax), copernicia cerifera(carnauba) wax, disteardimonium hectorite, dipalmitoyl hydroxyproline, silica silylate, octyldodecanol, menthoxypropanediol, cera microcristallina (microcrystalline wax), propylparaben, methylparaben, parfum (fragance), petrolatum, phospholipids, bht, cholesterol, polyglyceryl-2 dipolyhydroxystearate, euphorbia cerifera (candelilla) wax, cetearyl glucoside, cetearyl alcohol, glycosphingolipids. May contain: ci 77019 (mica), ci 77891 (titanium dioxide), ci 77491, ci 77492, ci 77499 (iron oxides), silica, ci 75470 (carmine), calcium aluminum borosilicate, tin oxide, ricinus communis (castor) seed oil, ci 42090 (blue 1 lake), talc, ci 15850:1 (red 7 lake), ci 19140 (yellow 5 lake), ci 45380:2 (red 21 lake), ci 45410:1 (red 27 lake).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Carton
-
INGREDIENTS AND APPEARANCE
LBEL EFFET PARFAIT ROUGE ALISSANT
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.24 g in 4 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.06 g in 4 g Inactive Ingredients Ingredient Name Strength myristyl myristate (UNII: 4042ZC00DY) yellow wax (UNII: 2ZA36H0S2V) carnauba wax (UNII: R12CBM0EIZ) hectorite (UNII: 08X4KI73EZ) dipalmitoyl hydroxyproline (UNII: E6AHA53N1H) silicon dioxide (UNII: ETJ7Z6XBU4) octyldodecanol (UNII: 461N1O614Y) propylparaben (UNII: Z8IX2SC1OH) methylparaben (UNII: A2I8C7HI9T) petrolatum (UNII: 4T6H12BN9U) butylated hydroxytoluene (UNII: 1P9D0Z171K) cholesterol (UNII: 97C5T2UQ7J) candelilla wax (UNII: WL0328HX19) cetostearyl alcohol (UNII: 2DMT128M1S) mica (UNII: V8A1AW0880) titanium dioxide (UNII: 15FIX9V2JP) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) ferrosoferric oxide (UNII: XM0M87F357) tin (UNII: 387GMG9FH5) castor oil (UNII: D5340Y2I9G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) aluminum oxide (UNII: LMI26O6933) talc (UNII: 7SEV7J4R1U) D&C RED NO. 7 (UNII: ECW0LZ41X8) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 21 (UNII: 08744Z6JNY) D&C RED NO. 27 (UNII: 2LRS185U6K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-011-62 1 in 1 BOX 1 NDC:13537-011-61 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/15/2010 Labeler - VENTURA INTERNATIONAL LTD., (602751344)