Label: PREHEVBRIO (hepatitis b vaccine- recombinant injection, suspension
- NDC Code(s): 75052-001-01, 75052-001-10
- Packager: VBI Vaccines (Delaware) Inc.
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PREHEVBRIO TM safely and effectively. See full prescribing information for PREHEVBRIO.
PREHEVBRIO [Hepatitis B Vaccine (Recombinant)]
Injectable suspension, for intramuscular use
Initial U.S. Approval: 2021INDICATIONS AND USAGE
PREHEVBRIO is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PREHEVBRIO is approved for use in adults 18 years of age and older. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
PREHEVBRIO is an injectable suspension, for intramuscular use supplied as a single-dose vial. A single dose of PREHEVBRIO is 1.0 mL ( 3)
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PREHEVBRIO. ( 4)
ADVERSE REACTIONS
Individuals 18 through 44 years of age: The most common local reactions following each dose of PREHEVBRIO were injection site pain (52.0 – 58.3%) and tenderness (52.6 – 59.6%). The most common systemic reactions following each dose of PREHEVBRIO were headache (17.2 – 25.8%), fatigue (20.1- 28.3%) and myalgia (22.2 – 29.9%).
Individuals 45 through 64 years of age: The most common local reactions following each dose of PREHEVBRIO were injection site pain (42.2 – 48.8%) and tenderness (43.2 – 50.5%). The most common systemic reactions following each dose of PREHEVBRIO were headache (13.8 – 21.3%), fatigue (14.3 – 19.7%) and myalgia (16.7 – 24.1%).
Individuals ≥ 65 years of age: The most common local reactions following each dose of PREHEVBRIO were injection site pain (26.7 – 34.8%) and tenderness (30.2 – 32.8%). The most common systemic reactions following each dose of PREHEVBRIO were headache (7.3 – 12.2%), fatigue (11.5 – 14.5%) and myalgia (11.5 - 16.6%). ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact VBI Vaccines at 1-888-421-8808 (toll-free) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov .
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Schedule
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions
5.2 Immunocompromised Individuals
5.3 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Immune Globulin
7.2 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Adults on Hemodialysis
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Evaluation of Immunogenicity
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage Conditions
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection.
2.1 Dosage and Schedule
Administer a series of three doses (1.0 mL each) of PREHEVBRIO on a 0-, 1- and 6-month schedule.
2.2 Administration
Shake the vial of PREHEVBRIO well to obtain a slightly opaque, white suspension.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
Administer PREHEVBRIO by intramuscular injection.
-
3 DOSAGE FORMS AND STRENGTHS
PREHEVBRIO is an injectable suspension, for intramuscular use supplied as a single-dose vial. A single dose of PREHEVBRIO is 1.0 mL [see How Supplied/Storage and Handling ( 16.1)].
-
4 CONTRAINDICATIONS
Do not administer PREHEVBRIO to individuals with a history of severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PREHEVBRIO [see Description ( 11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of PREHEVBRIO.
-
6 ADVERSE REACTIONS
Individuals 18 through 44 years of age: The most common local reactions following each dose of PREHEVBRIO were injection site pain (52.0 – 58.3%) and tenderness (52.6 – 59.6%). The most common systemic reactions following each dose of PREHEVBRIO were headache (17.2 – 25.8%), fatigue (20.1- 28.3%) and myalgia (22.2 – 29.9%).
Individuals 45 through 64 years of age: The most common local reactions following each dose of PREHEVBRIO were injection site pain (42.2 – 48.8%) and tenderness (43.2 – 50.5%). The most common systemic reactions following each dose of PREHEVBRIO were headache (13.8 – 21.3%), fatigue (14.3 – 19.7%) and myalgia (16.7 – 24.1%).
Individuals ≥ 65 years of age: The most common local reactions following each dose of PREHEVBRIO were injection site pain (26.7 – 34.8%) and tenderness (30.2 – 32.8%). The most common systemic reactions following each dose of PREHEVBRIO were headache (7.3 – 12.2%), fatigue (11.5 – 14.5%) and myalgia (11.5 - 16.6%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of PREHEVBRIO was evaluated in 2 active-controlled clinical studies (Studies 1 and 2) involving 4,443 subjects who received at least 1 dose of PREHEVBRIO (n = 2,920) or Engerix-B [Hepatitis B Vaccine (Recombinant)] (n = 1,523) administered according to a 0-, 1- and 6-months schedule.
Study 1 in adults ≥18 years of age
Study 1 was a randomized, double-blind, active-controlled, multicenter study that enrolled subjects in the United States (US), Canada, Belgium and Finland in which 796 subjects received at least 1 dose of PREHEVBRIO and 811 subjects received at least 1 dose of Engerix-B. In the total study population at baseline the mean age was 57 years, 81% were age ≥45 years; 62% were women; 90% were White, 8% Black, 1% Asian, and 10% Hispanic/Latino; 37% were obese (body mass index [BMI] >30 kg/m 2), 14% were current smokers and 8% had Type 2 diabetes mellitus. Demographic and baseline characteristics were similar in both vaccine groups.
Solicited Local and Systemic Adverse Reactions
Subjects were monitored for local and systemic adverse reactions using diary cards for a 7-day period starting on the day of vaccination. The percentages of subjects who reported local and systemic reactions in Study 1 are shown by age subgroup in Table 1 to Table 3.
Table 1: Study 1: Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination (18 through 44 years of age)
PREHEVBRIO
Dose 1
(N=145)
%PREHEVBRIO
Dose 2
(N=141)
%PREHEVBRIO
Dose 3
(N= 134)
%Engerix-B
Dose 1
(N=154)
%Engerix-B
Dose 2
(N=152)
%Engerix-B
Dose 3
(N=148)
%a Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization
b Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization
c Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization
d Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis
e Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis.
f Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
Local Reaction Pain 58.6 50.4 46.3 33.8 28.9 31.8 Pain, Grade 3 or greater a 0 0 0 0 0 0 Tenderness 53.8 50.4 42.5 32.5 32.2 36.5 Tenderness, Grade 3 or greater b 0.7 0 0.7 0.6 0.7 0.7 Itching 2.1 3.5 6.0 7.1 3.9 7.4 Itching, Grade 3 or greater c 0 0 0 0 0.7 1.4 Redness (≥ 2.5 cm) 0.7 1.4 1.5 0.6 1.3 0 Redness, Grade 3 or greater d 0 0 0 0 0 0 Swelling (≥ 2.5 cm) 2.8 1.4 0.7 0 1.3 2.0 Swelling, Grade 3 or greater e 0 0 0 0 0.7 1.4 Systemic Reaction Headache 33.8 24.1 20.9 29.9 19.1 13.5 Headache, Grade 3 or greater a 1.4 0.7 0 1.3 0.7 0 Fatigue 29.7 22.0 22.4 31.8 20.4 20.3 Fatigue, Grade 3 or greater c 1.4 0.7 0 0.6 2.0 1.4 Myalgia 27.6 24.1 21.6 20.8 11.8 10.1 Myalgia, Grade 3 or greater c 0.7 0 0 0 1.3 0 Diarrhea 9.7 5.7 4.5 9.7 5.9 7.4 Diarrhea, Grade 3 or greater f 0.7 0 0 0 0.7 0 Nausea/Vomiting 8.3 4.3 4.5 7.8 6.6 6.1 Nausea/Vomiting, Grade 3 or greater f 0 0.7 0 0 0.7 0 Fever (≥100.4°F) 0.7 0.7 0 1.3 0 0.7 Fever, Grade 3 or greater (≥102.1°F) 0.7 0 0 0 0 0 Table 2: Study 1: Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination (45 through 64 years of age) PREHEVBRIO
Dose 1
(N=355)
%PREHEVBRIO
Dose 2
(N=350)
%PREHEVBRIO
Dose 3
(N=343)
%Engerix-B
Dose 1
(N=361)
%Engerix-B
Dose 2
(N=357)
%Engerix-B
Dose 3
(N=349)
%a Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization
b Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization
c Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization
d Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis
e Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis.
f Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
Local Reaction Pain 46.8 44.9 39.4 22.2 15.4 17.2 Pain, Grade 3 or greater a 0 0 0.3 0 0 0 Tenderness 48.7 42.6 40.5 23.8 16.5 17.5 Tenderness, Grade 3 or greater b 0.8 0.6 0.3 0 0 0.3 Itching 4.5 3.1 3.8 3.9 2.0 3.4 Itching, Grade 3 or greater c 0 0.3 0 0 0 0 Redness (≥ 2.5 cm) 1.7 0.6 0.3 1.1 0.3 1.1 Redness, Grade 3 or greater d 0 0 0 0.8 0.3 0.6 Swelling (≥ 2.5 cm) 1.4 0.3 0.9 0 0.6 0.3 Swelling, Grade 3 or greater e 0 0 0.3 0 0 0 Systemic Reaction Headache 21.4 13.7 15.7 20.5 11.2 14.0 Headache, Grade 3 or greater a 0 0 0.3 0.3 0.3 0.3 Fatigue 16.6 16.9 12.5 22.2 11.5 12.3 Fatigue, Grade 3 or greater c 0.6 0 0.3 0.6 0.3 0.6 Myalgia 21.4 20.0 15.5 16.1 8.4 9.5 Myalgia, Grade 3 or greater c 0.6 0 0 0 0 0 Diarrhea 4.8 4.0 3.2 6.4 3.6 3.7 Diarrhea, Grade 3 or greater f 0 0 0 0 0 0 Nausea/Vomiting 4.2 2.9 2.3 6.4 3.6 2.6 Nausea/Vomiting, Grade 3 or greater f 0 0 0 0 0 0 Fever (≥100.4°F) 0.6 0 0 0.3 0.3 0.6 Fever, Grade 3 or greater (≥102.1°F) 0 0 0 0 0 0.3 Table 3: Study 1: Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination (≥ 65 years of age)
PREHEVBRIO
Dose 1
(N=296)
%PREHEVBRIO
Dose 2
(N=288)
%PREHEVBRIO
Dose 3
(N=281)
%Engerix-B
Dose 1
(N=296)
%Engerix-B
Dose 2
(N=292)
%Engerix-B
Dose 3
(N= 288)
%a Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization
b Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization
c Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization
d Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis
e Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis.
f Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
Local Reaction Pain 34.8 28.8 26.7 16.2 12.0 11.1 Pain, Grade 3 or greater a 0 0 0 0.3 0 0 Tenderness 32.8 30.2 31.0 14.2 12.0 10.1 Tenderness, Grade 3 or greater b 0 0 0 0 0 0 Itching 6.1 3.8 5.0 4.1 1.4 2.4 Itching, Grade 3 or greater c 0 0 0 0 0 0 Redness (≥ 2.5 cm) 1.0 0.3 1.4 0.7 0.3 0 Redness, Grade 3 or greater d 0.3 0 0.4 0 0.3 0 Swelling (≥ 2.5 cm) 1.0 0.7 1.1 1.4 0.3 0.3 Swelling, Grade 3 or greater e 0.3 0 0 0 0.3 0 Systemic Reaction Headache 12.2 7.3 7.8 12.8 5.8 6.9 Headache, Grade 3 or greater a 0 0 0 0 0 0 Fatigue 14.5 11.5 12.5 17.9 9.9 10.1 Fatigue, Grade 3 or greater c 0 0 0 0.7 0 0.3 Myalgia 16.6 11.5 13.2 12.8 8.2 6.9 Myalgia, Grade 3 or greater c 0 0 0 0 0.3 0 Diarrhea 6.4 4.2 1.1 6.4 2.4 3.5 Diarrhea, Grade 3 or greater f 0.3 0 0 0.3 0 0 Nausea/Vomiting 3.7 0.7 1.1 1.7 1.7 0.7 Nausea/Vomiting, Grade 3 or greater f 0 0 0 0.3 0 0.3 Fever (≥100.4°F) 0 0 0.7 0 0 0.7 Fever, Grade 3 or greater (≥102.1°F) 0 0 0 0 0 0 The median duration of local and systemic solicited adverse reactions was 1-2 days in both treatment groups. Among all subjects who received PREHEVBRIO, the frequencies of the most commonly reported solicited reactions extending beyond the 7-day assessment period were as follows: fatigue (4.1%), injection site pain (2.0%), headache (1.9%) and myalgia (1.9%).
Study 2 in adults 18 through 45 years of age
Study 2 was a randomized, double-blind, active-controlled, multicenter study that enrolled subjects in the US, Canada, Belgium, Finland, Germany and the United Kingdom in which 2,124 subjects received at least 1 dose of PREHEVBRIO and 712 subjects received at least 1 dose of Engerix-B. In the total study population at baseline, the mean age was 34 years; 58% were women; 92% were White, 6% Black, 2% Asian, and 10% Hispanic/Latino; 18% were obese (BMI >30 kg/m 2) and 19% were current smokers. Demographic and baseline characteristics were similar in both vaccine groups.
Solicited Local and Systemic Adverse Reactions
Subjects were monitored for local and systemic adverse reactions using diary cards for a 7-day period starting on the day of vaccination. The percentages of subjects who reported local and systemic reactions in Study 2 are shown in Table 4.
Table 4: Study 2: Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination (18 through 45 years of age)
PREHEVBRIO
Dose 1
(N=2122) a
%PREHEVBRIO
Dose 2
(N=2071)
%PREHEVBRIO
Dose 3
(N=1967)
%Engerix-B
Dose 1
(N=712)
%Engerix-B
Dose 2
(N=701)
%Engerix-B
Dose 3
(N=671)
%a Two subjects without solicited adverse event data following dose 1 of PREHEVBRIO were excluded from this analysis.
b Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization
c Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization
d Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization
e Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis
f Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis
g Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
Local Reaction Pain 58.2 52.2 52.5 35.1 29.2 32.5 Pain, Grade 3 or greater b 0.3 0.3 0.4 0.1 0 0.3 Tenderness 59.9 52.9 55.5 37.6 30.4 33.8 Tenderness, Grade 3 or greater c 0.8 0.9 0.8 0.6 0.1 0.1 Itching 5.7 5.7 6.7 6.6 5.3 5.4 Itching, Grade 3 or greater d 0 0 0.1 0.3 0.1 0 Redness (≥ 2.5 cm) 1.1 1.1 1.3 0.6 0.4 1.0 Redness, Grade 3 or greater e 0.2 0 0.2 0.1 0.1 0.1 Swelling (≥ 2.5 cm) 1.2 0.9 1.1 0.6 0 0.4 Swelling, Grade 3 or greater f 0.1 0 0.1 0 0 0 Systemic Reaction Headache 25.1 16.7 17.4 24.2 15.0 18.3 Headache, Grade 3 or greater b 0.3 0.2 0.5 0.4 0.4 0.6 Fatigue 28.4 19.8 20.2 27.1 17.8 22.1 Fatigue, Grade 3 or greater d 0.5 0.8 0.6 0.4 0.6 0.6 Myalgia 30.3 21.9 23.6 17.7 13.0 18.5 Myalgia, Grade 3 or greater d 0.3 0.6 0.5 0.4 0.1 0.4 Diarrhea 7.4 5.0 4.4 9.6 4.9 5.4 Diarrhea, Grade 3 or greater g 0.2 0.1 0.1 0 0 0 Nausea/Vomiting 6.7 3.7 4.7 7.0 3.6 3.9 Nausea/Vomiting, Grade 3 or greater g 0 0 0.2 0 0.1 0 Fever (≥100.4°F) 0.3 0.3 0.6 0.4 0.3 0.9 Fever, Grade 3 or greater (≥102.1°F) 0 0.1 0.1 0.1 0 0 The median duration of local and systemic solicited adverse reactions was 1-2 days in both treatment groups. Among all subjects who received PREHEVBRIO, the frequencies of the most commonly reported solicited reactions extending beyond the 7-day assessment period were as follows: fatigue (3.5%), injection site pain (2.0%), headache (1.9%) and myalgia (1.8%).
Unsolicited Adverse Events (AEs)
In both studies, unsolicited adverse events, including serious and non-serious events, that occurred within 28 days following each vaccination were recorded on a diary card by all subjects.
In both studies combined, unsolicited AEs that occurred within 28 days of any vaccination were reported by 48.3% and 48.4% of subjects who received PREHEVBRIO or Engerix-B, respectively. Unsolicited AEs in subjects who received PREHEVBRIO for which available information suggests a causal relationship to vaccination include injection site bruising (1.4%), dizziness/vertigo (1.1%), general pruritus/itchiness (0.2%), arthralgia (0.2%), urticaria/hives (0.2%) and lymphadenopathy/lymph node pain (0.1%).
Serious Adverse Events (SAEs)
In both studies, SAEs were collected from first vaccination through 6 months following the last vaccination. In both studies combined, SAEs were reported by 0.9% and 0.6% within 28 days of vaccination with PREHEVBRIO or Engerix-B, respectively. SAEs were reported by 2.5% of subjects in the PREHEVBRIO group and 1.6% in the Engerix-B group from the first vaccination through 6 months following the third vaccination. There were no notable patterns or numerical imbalances between vaccination groups for specific categories of serious adverse events that would suggest a causal relationship to PREHEVBRIO.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to PREHEVBRIO during pregnancy. Women who receive PREHEVBRIO during pregnancy are encouraged to contact 1-888-421-8808 (toll-free).
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In clinically recognized pregnancies in the US general population, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20%.
There are no adequate and well-controlled studies of PREHEVBRIO in pregnant women. Available human data on PREHEVBRIO administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study has been performed in female rats administered the equivalent of a single human dose of PREHEVBRIO on four occasions; twice prior to mating, twice during gestation. The study revealed no evidence of harm to the fetus due to the vaccine [see Animal Data below].
Animal Data
A developmental toxicity study has been performed in female rats using a dose equivalent to the adult human dose. In the study, female rats received 0.5 mL (2 x 0.25 mL injections) of a vaccine formulation containing 10 mcg HBsAg (S, pre-S1, pre-S2) adsorbed on to aluminum hydroxide by intramuscular injection 30 days and 15 days prior to mating and on gestation days 4 and 15. No adverse effects of pre-weaning development were observed. There was no evidence of fetal malformations or variations.
8.2 Lactation
Risk Summary
It is not known whether PREHEVBRIO is excreted in human milk. Data are not available to assess the effects of PREHEVBRIO on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PREHEVBRIO and any potential adverse effects on the breastfed child from PREHEVBRIO or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness of PREHEVBRIO have not been established in individuals less than 18 years of age.
8.5 Geriatric Use
Study 1 included 296 adults 65 through 86 years of age who received PREHEVBRIO. Among subjects who received PREHEVBRIO, a seroprotective level of antibody to HBsAg was achieved in 83.6% of those ≥ 65 years of age compared to 94.8% in adults 45 through 64 years of age and 99.2% in adults 18 through 44 years of age [see Evaluation of Immunogenicity ( 14.1)].
Frequencies of local and systemic solicited adverse reactions were generally lower in elderly subjects ≥65 years of age than in younger subjects [see Adverse Reactions ( 6)].
-
11 DESCRIPTION
PREHEVBRIO [Hepatitis B Vaccine (Recombinant)] is a sterile suspension for intramuscular injection.
PREHEVBRIO contains the small (S), middle (pre-S2) and large (pre-S1) hepatitis B surface antigens, co-purified from genetically modified CHO (Chinese Hamster Ovary) cells cultured in growth medium containing vitamins, amino acids, minerals, and fetal bovine serum.
The hepatitis B surface antigens are co-purified from the supernatant of CHO cells by a series of physicochemical steps as virus-like particles containing CHO cell membrane lipids.
Each 1.0 mL dose is formulated to contain 10 mcg hepatitis B surface antigens (S, pre-S1 and pre-S2) adsorbed on aluminum hydroxide [Al(OH) 3] as an adjuvant (aluminum content of 0.5 mg/mL).
Each 1.0 mL dose of PREHEVBRIO also contains sodium chloride (NaCl) (8.45 mg/dose), potassium chloride (KCl) (0.02 mg/dose), disodium hydrogen phosphate dodecahydrate (Na 2HPO 4.12H 2O) (0.38 mg/dose), potassium dihydrogen phosphate anhydrous (KH 2PO 4) (0.02 mg/dose) and water for injections (WFI). Each dose may contain residual amounts of CHO cell proteins (up to 2.5 ng/dose), CHO cell DNA (up to 10 pg/dose), Bovine Serum Albumin (up to 2.5 ng/dose) and Formaldehyde (up to 500 ng/dose) from the manufacturing process.
PREHEVBRIO does not contain a preservative.
The vial stoppers are not made with natural rubber latex.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
PREHEVBRIO has not been evaluated for carcinogenic, mutagenic potential or male infertility in animals. In a developmental toxicity study in rats with a vaccine formulation containing 10 mcg HBsAg (S, pre-S1, pre-S2) adsorbed on to aluminum hydroxide there were no effects on female fertility [see Animal Data ( 8.1)].
-
14 CLINICAL STUDIES
14.1 Evaluation of Immunogenicity
The immunogenicity of PREHEVBRIO was evaluated in comparison with a US-licensed hepatitis B vaccine (Engerix-B) in 2 randomized, active controlled, double-blind, multi-center Phase 3 clinical trials in adults. PREHEVBRIO and Engerix-B were administered according to a 0-, 1- and 6-month schedule. For subject baseline characteristics, see section 6.1.
The trials compared the seroprotection rates (SPR), defined as the proportion of participants with anti-HBs titers ≥ 10 mIU/mL, induced by PREHEVBRIO and Engerix-B. Non-inferiority was met if the lower bound of the 95% confidence interval (CI) of the difference in SPR (PREHEVBRIO minus Engerix-B) was greater than -5%.
Study 1 in adults ≥18 years of age
The immunogenicity population included 718 subjects who received PREHEVBRIO and 723 subjects who received Engerix-B. The mean age was 57 years in both groups. The primary analysis compared the SPR, 4 weeks after receiving the third dose of PREHEVBRIO or Engerix-B in subjects ≥ 18 years of age. The SPR induced by PREHEVBRIO compared to Engerix-B was non-inferior in subjects ≥ 18 years of age ( Table 5).
Table 5: Study 1: Seroprotection Rate (SPR) 4 Weeks After Receiving the Third Dose of PREHEVBRIO or Engerix-B Abbreviations: N=number of subjects in the analysis set; SPR= Seroprotection Rate (percent of subjects with anti-HBs titers ≥10 mIU/mL)
a Per-protocol set (PPS). PPS included all subjects in the full analysis set who received all 3 vaccinations, had an evaluable serum immunogenicity sample at baseline and at the time point of interest, were seronegative at baseline, and had no major protocol violations leading to exclusion.
b Full analysis set (FAS). FAS included all subjects who received at least 1 vaccination and provided at least 1 evaluable serum immunogenicity sample both at baseline and after baseline. Subjects were seronegative at baseline.
c Non-inferiority was met because the lower bound of the 95% CI of the difference in SPR (PREHEVBRIO - Engerix-B) was > -5%.
d The SPR following PREHEVBRIO was statistically significantly higher than following Engerix-B (lower bound of the 95% CI of the difference in SPR was > 0%).
e Exploratory analysis
Study Population PREHEVBRIO
NPREHEVBRIO
SPR (95% CI)
Engerix-B
NEngerix-B
SPR (95% CI)
Difference in SPR;
PREHEVBRIO – Engerix-B (95% CI)All Adults (Age 18+) a 718 91.4 (89.1, 93.3) 723 76.5 (73.2, 79.5) 14.9 (11.2, 18.6) c Age 45+ b 625 89.4 (86.8, 91.7) 627 73.1 (69.4, 76.5) 16.4 (12.2, 20.7) d Age 18-44 125 99.2 (95.6, 100.0) 135 91.1 (85.0. 95.3) - e Age 45-64 325 94.8 (91.8, 96.6) 322 80.1 (75.3, 84.3) - e Age 65 + 268 83.6 (78.6, 87.8) 266 64.7 (58.6, 70.4) - e Study 2 in adults 18 through 45 years of age
The immunogenicity population included 1,753 subjects who received PREHEVBRIO and 592 subjects who received Engerix-B. The mean age was 34 years in the PREHEVBRIO group and 33 years in the Engerix-B group. The study compared the SPR, 4 weeks after receiving the third dose of PREHEVBRIO or Engerix-B in all subjects. The SPR induced by PREHEVBRIO compared to Engerix-B was non-inferior ( Table 6).
Table 6: Study 2: Seroprotection Rate (SPR) 4 Weeks After Receiving the Third Dose of PREHEVBRIO or Engerix-B SPR= Seroprotection Rate (percent of subjects with anti-HBs titers ≥10 mIU/mL)
*Non-inferiority was met because the lower bound of the 95% CI of the difference in SPR (PREHEVBRIO - Engerix-B) was > -5%.
Study Population PREHEVBRIO
NPREHEVBRIO
SPR (95% CI)Engerix-B
NEngerix-B
SPR (95% CI)
Difference in SPR;
PREHEVBRIO –Engerix-B
(95% CI)Age 18-45 1753 99.3 (98.7. 99.6) 592 94.8 (92.7, 96.4) 4.5 (2.9, 6.6) * - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Inform vaccine recipient of the potential benefits and risks associated with vaccination with PREHEVBRIO, as well as the importance of completing the immunization series.
- Emphasize that PREHEVBRIO contains non-infectious purified HBsAg and cannot cause hepatitis B infection.
- Advise vaccine recipient to report any adverse events to their healthcare provider or to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 and www.vaers.hhs.gov.
- Provide the Vaccine Information Statements, which are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
Manufactured by: VBI Vaccines Inc.
160 Second St., 3rd Floor, Cambridge, MA, USA, 02142
U.S. License No. 2219
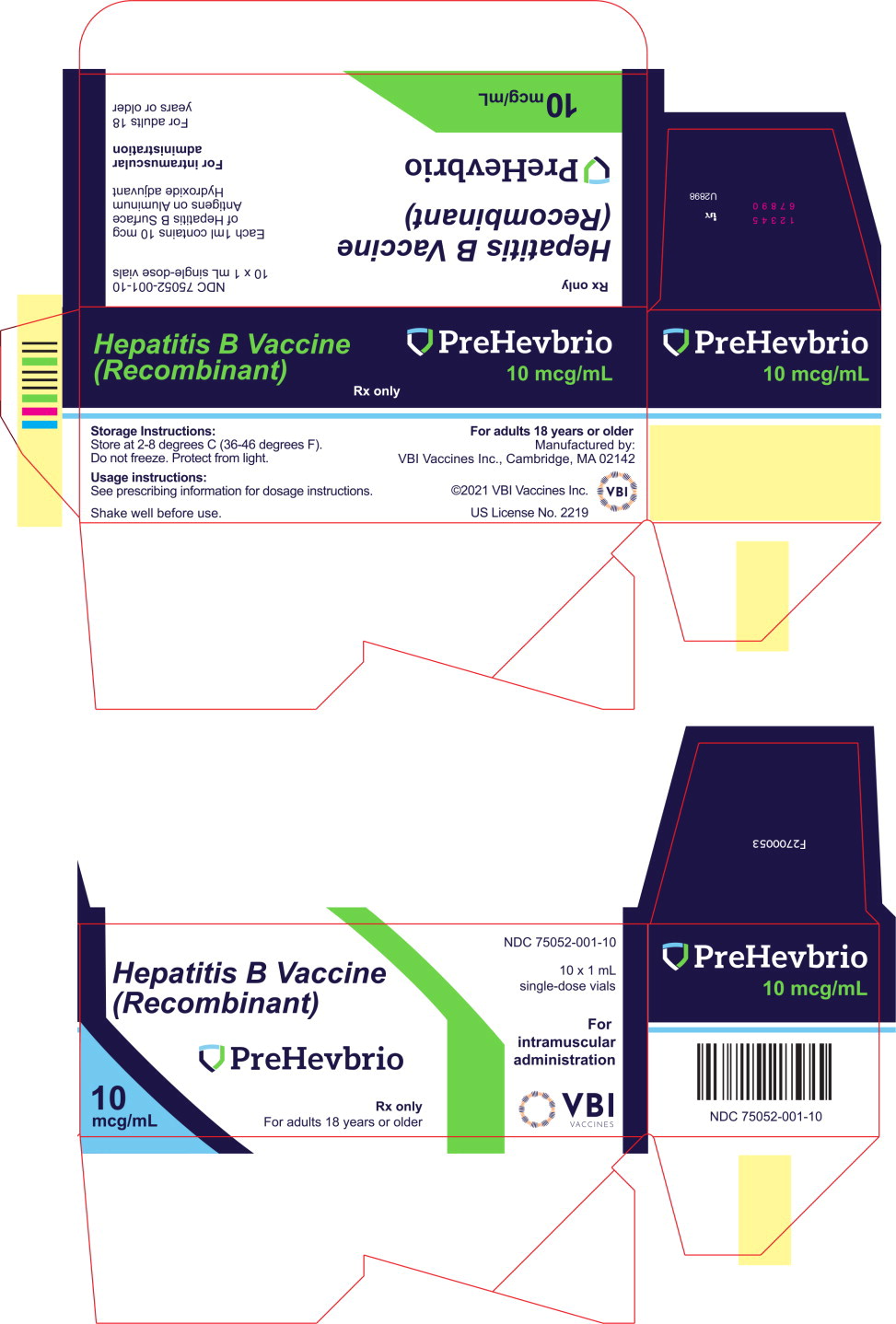
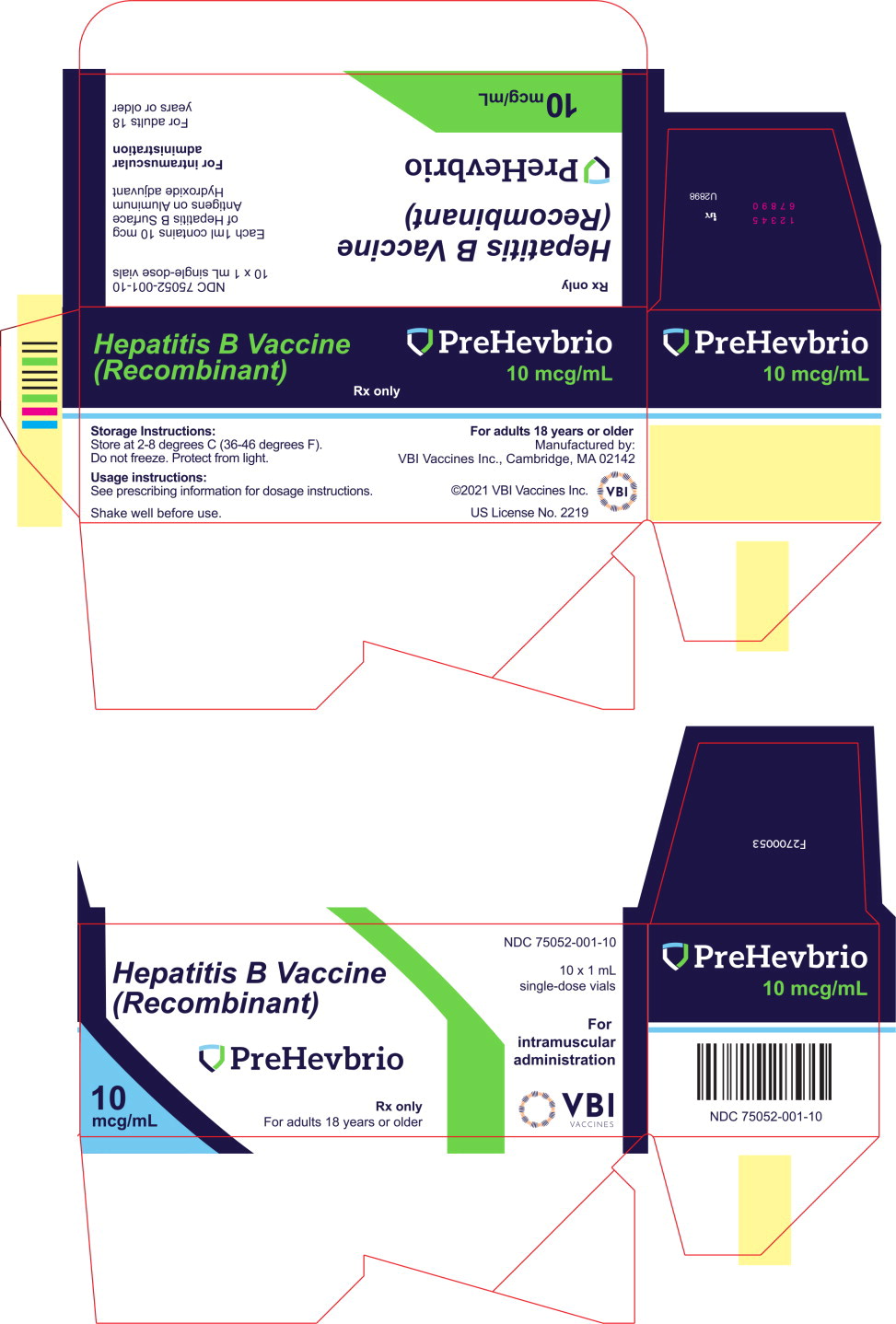
©2021 VBI Vaccines. All rights reserved. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREHEVBRIO
hepatitis b vaccine (recombinant) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:75052-001 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM S) (UNII: 2U8266YW9L) (RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM S) - UNII:2U8266YW9L) RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM S) 10 ug in 1 mL RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM M) (UNII: SND8HL4KQG) (RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM M) - UNII:SND8HL4KQG) RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM M) 1.1 ug in 1 mL RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM L) (UNII: C6PFS5DX5Y) (RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM L) - UNII:C6PFS5DX5Y) RECOMBINANT HEPATITIS B SURFACE ANTIGEN (ISOFORM L) 0.6 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8450 ug in 1 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) 20 ug in 1 mL SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) 380 ug in 1 mL MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) 20 ug in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) 1500 ug in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75052-001-10 10 in 1 CARTON 1 NDC:75052-001-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125737 03/02/2022 Labeler - VBI Vaccines (Delaware) Inc. (103812236) Establishment Name Address ID/FEI Business Operations BioReliance 505004556 analysis Establishment Name Address ID/FEI Business Operations SciVac Ltd 514477301 api manufacture, analysis, pack, label, manufacture Establishment Name Address ID/FEI Business Operations Envigo CRS (Israel) Limited 514627793 analysis Establishment Name Address ID/FEI Business Operations Hy-Labs 600013676 analysis Establishment Name Address ID/FEI Business Operations IMI TAMI Institute for Research and Development Ltd. 600045280 analysis