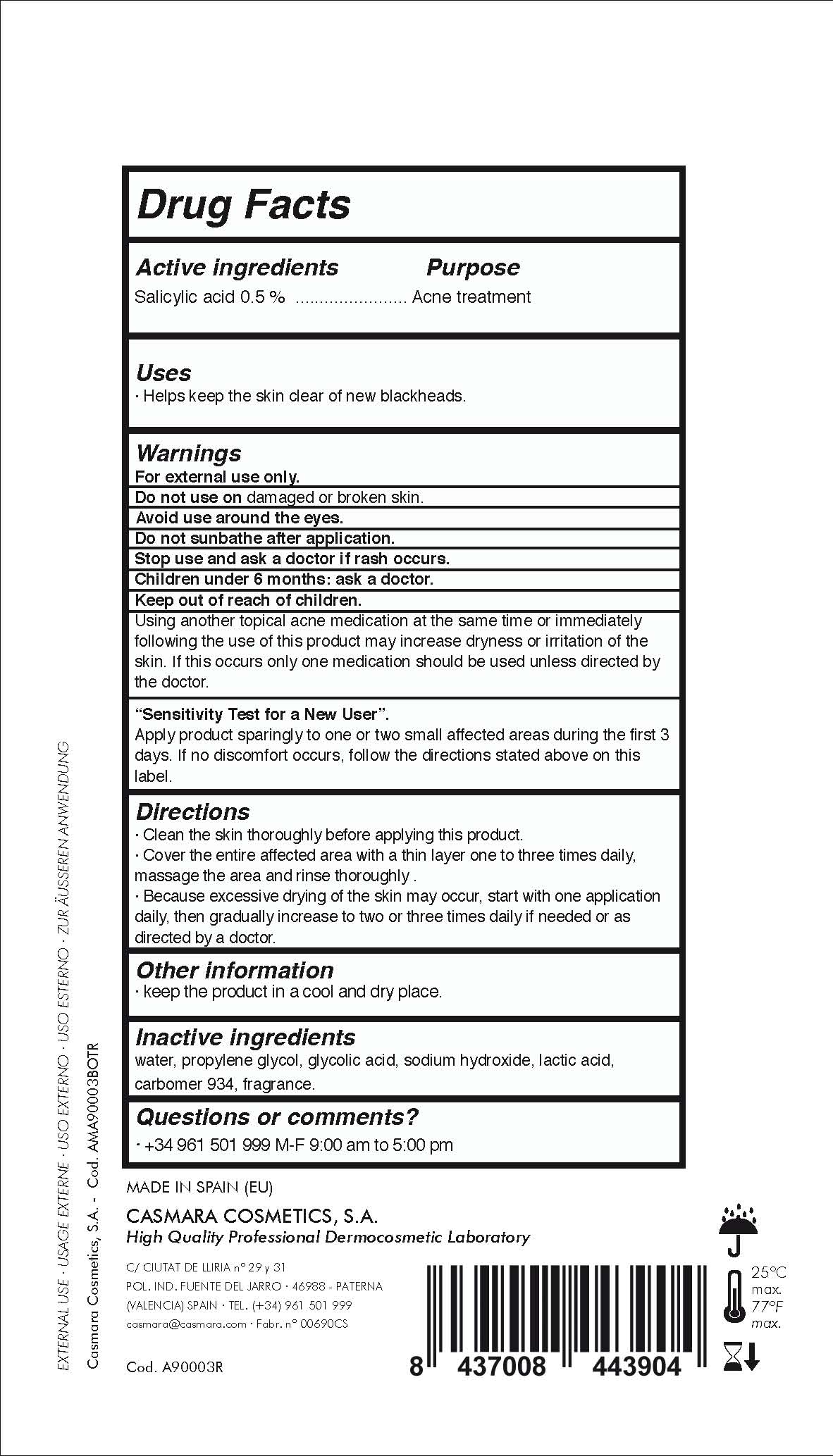
Label: NATURAL PEELING TRIACTIVE ACNE- salicylic acid lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 20151-077-01 - Packager: Casmara Cosmetics, SA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 1, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- Questions or Comments?
- OTHER SAFETY INFORMATION
-
Directions
. clean the skin thoroughly before applying this product
. cover the entire affected area with a thin layer one to three times daily, massage the area and rinse thoroughly.
. because excessive dryness of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
-
DOSAGE & ADMINISTRATION
. clean the skin thoroughly before applying this product
. cover the entire affected area with a thin layer one to three times daily, massage the area and rinse thoroughly.
. because excessive dryness of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- Uses
- PURPOSE
- Active Ingredients
-
WARNINGS
For external use only.
Do not use on damaged or broken skin.
Avoid use around the eyes
Do not sunbathe after application
Stop use and ask a doctor if rash occurs.
Children under 6 months: as a doctor.
KEEP OUT OF THE REACH OF CHILDREN
USING OTHER TOPICAL ACNE DRUGS AT THE SAME TIME OR INMEDIATELY FOLLOWING THE USE OF THIS PRODUCT MAY INCREASE DRYNESS OR IRRITATION OF THE SKIN. IF THIS OCCURS, ONLY ONE MEDICATION SHOULD BE USED UNLESS DIRECTED BY A DOCTOR
- Sensitive Test for a New User
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATURAL PEELING TRIACTIVE ACNE
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:20151-077 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 48.5 mg in 1 mL PERFLUNAFENE (UNII: 54A06VV62N) 0.1 mg in 1 mL CARBOMER 934 (UNII: Z135WT9208) 1.5 mg in 1 mL PROPANEDIOL (UNII: 5965N8W85T) 40 mg in 1 mL GLYCOLIC ACID (UNII: 0WT12SX38S) 5.6 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) 2 mg in 1 mL LACTIC ACID (UNII: 33X04XA5AT) 1.8 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:20151-077-01 500 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 07/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 07/03/2016 Labeler - Casmara Cosmetics, SA (464973544) Registrant - Casmara Cosmetics, SA (464973544) Establishment Name Address ID/FEI Business Operations Casmara Cosmetics, SA 464973544 manufacture(20151-077)