Label: GUAIFENESIN liquid
- NDC Code(s): 81033-102-05, 81033-102-10, 81033-102-51, 81033-102-52
- Packager: KESIN PHARMA CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Guaifenesin
- Description
- Inactive Ingredients
- Uses
- Warnings
- ASK DOCTOR
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Follow dosage below or use as directed by a physician.
Do not take more than 6 doses in any 24-hour periodAge Dose Adults and children 12 years and over 10 to 20mL (2 to 4 teaspoonfuls) every 4 hours Children 6 years to under 12 years 5 to 10mL (1 to 2 teaspoonfuls) every 4 hours Children 2 to under 6 years of age 2.5 to 5mL (1/2 to 1 teaspoonful) every 4 hours Children under 2 years of age Consult a physician -
SPL UNCLASSIFIED SECTION
Guaifenesin Oral Solution is a clear, colorless solution with a grape flavor, free of visible foreign matter supplied in the following oral dosage forms:
NDC 81033-102-05 Guaifenesin 100mg/5mL (Unit Dose Cup 5mL)
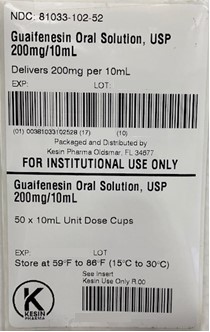
NDC 81033-102-10 Guaifenesin 200mg/10mL (Unit Dose Cup 10mL)
STORAGE
Keep tightly closed. Store at 15-30°C (59-86°F) - QUESTIONS
- PRINCIPAL DISPLAY PANEL - 5 mL Unit Dose Cup Label
- PRINCIPAL DISPLAY PANEL - 10 mL Unit Dose Cup Label
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81033-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN (UNII: A2I8C7HI9T) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) POTASSIUM CITRATE ANHYDROUS (UNII: 86R1NVR0HW) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81033-102-51 50 in 1 CARTON 11/20/2023 1 NDC:81033-102-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 2 NDC:81033-102-52 50 in 1 CARTON 11/20/2023 2 NDC:81033-102-10 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/20/2023 Labeler - KESIN PHARMA CORPORATION (117447816) Establishment Name Address ID/FEI Business Operations Kesin Pharma 117447816 pack(81033-102) , label(81033-102) Establishment Name Address ID/FEI Business Operations Wittman Pharma, Inc. 830980947 manufacture(81033-102)