Label: MAJOR- nasal decongestant spray
- NDC Code(s): 0904-7006-35
- Packager: MAJOR PHARMACEUTICALS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
-
When using this product
- ▪
- do not use more than directed
- ▪
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- ▪
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- ▪
- use of this container by more than one person may spread infection.
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- ▪
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- ▪
- children under 6 years of age: ask a doctor
To use: Push firmly down on cap and turn counter clockwise.
To Spray: Squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use. Secure cap after use.
- Other information
- Inactive ingredients
- Questions or comments?
-
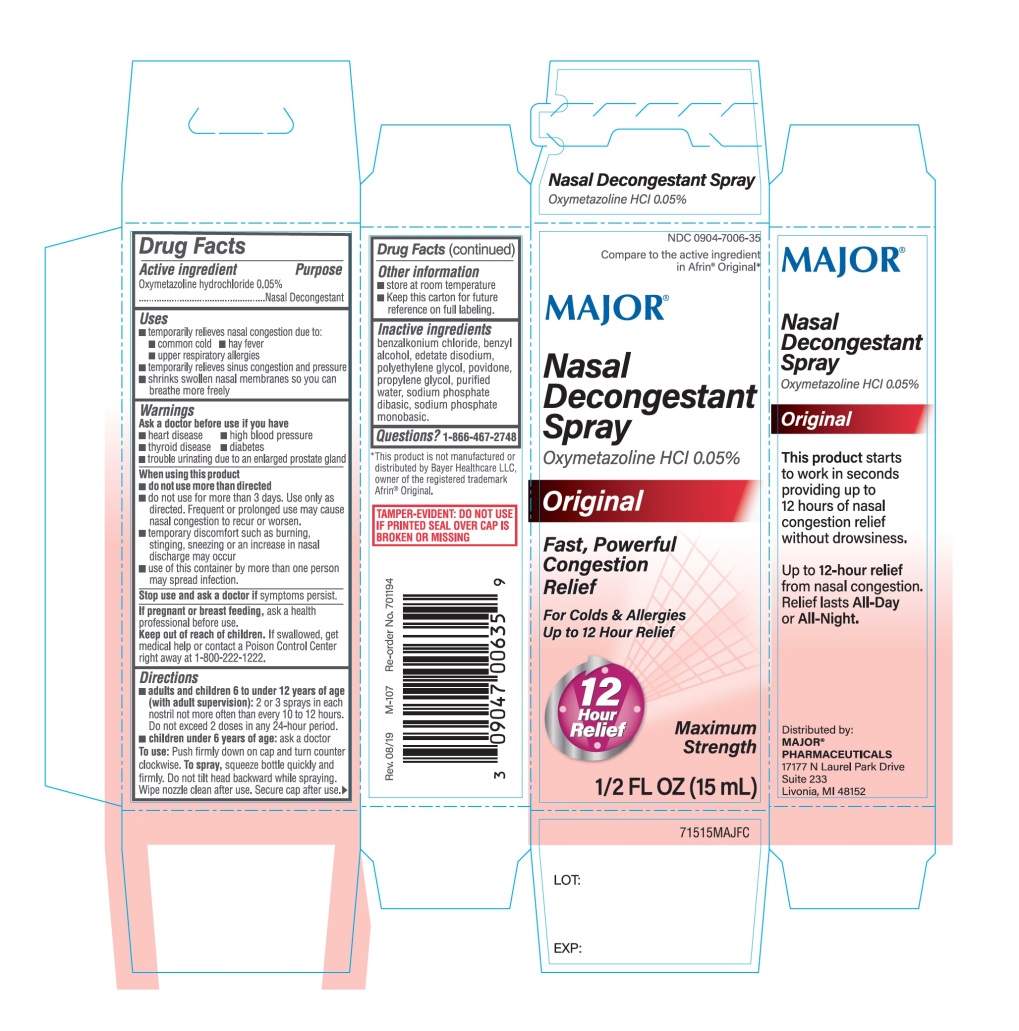
Principal Display Panel
MAJOR®
Compare to the active ingredient in Afrin® Original*
NDC# 0904-7006-35
Nasal Decongestant Spray
OXYMETAZOLINE HCL 0.05%
Original
Fast, Powerful Congestion Relief
For Colds & Allergies
Up to 12 Hour Relief
12 Hour Relief
Maximum Strength
NASAL DECONGESTANT
12 HOUR
1/2 FL OZ (15 mL)
TAMPER-EVIDENT: DO NOT USE IF PRINTED SEAL OVER CAP IS BROKEN OR MISSING.
Distributed by:
MAJOR® PHARMACEUTICALS
17177 N Laurel Park Drive
Suite 233
Livonia, MI 48152
This product starts to work in seconds providing up to 12 hours of nasal congestion relief without drowsiness.
Up to 12-hour relief from nasal congestion, Relief lasts All-Day or All-Night.
*This product is not manufactured or distributed by Bayer Healthcare LLC, owner of the registered trademark Afrin® Original.
-
INGREDIENTS AND APPEARANCE
MAJOR
nasal decongestant sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-7006 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-7006-35 1 in 1 CARTON 05/16/2019 1 15 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/16/2019 Labeler - MAJOR PHARMACEUTICALS (191427277)